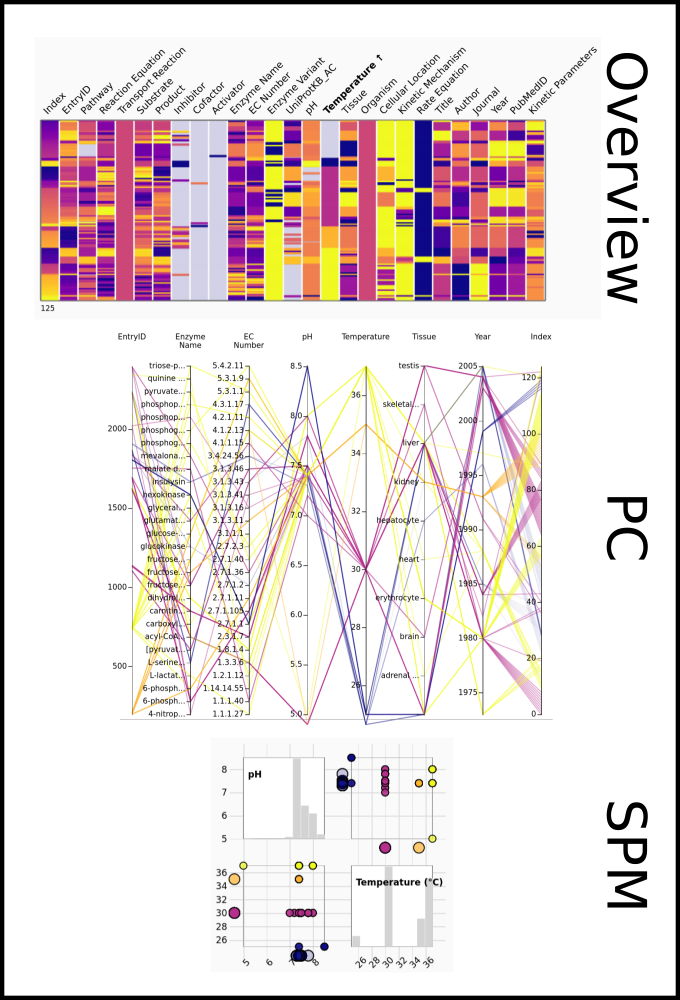
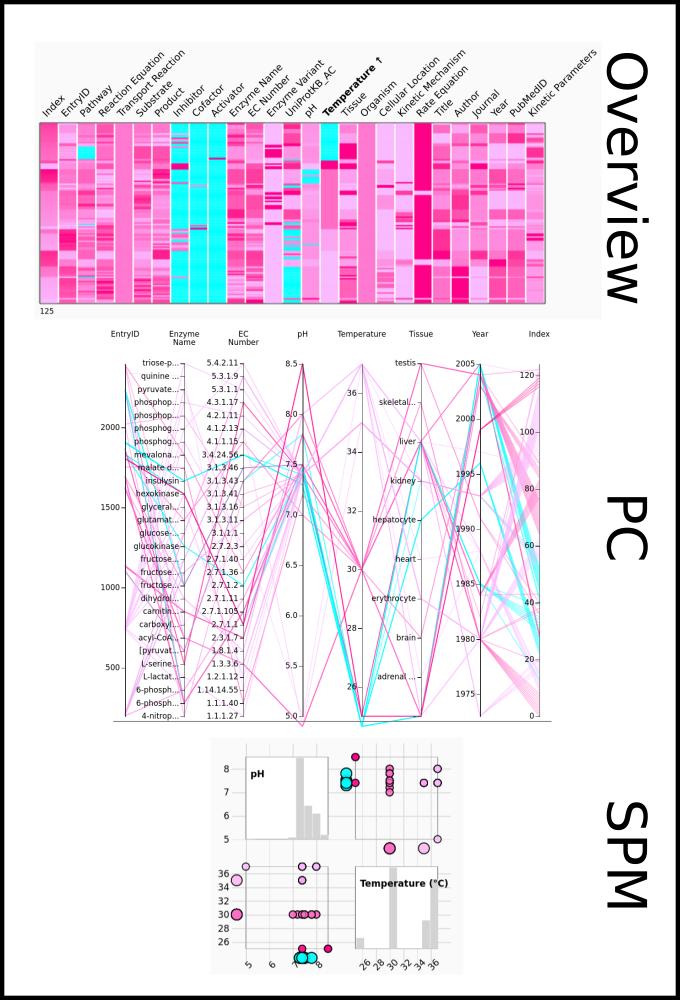
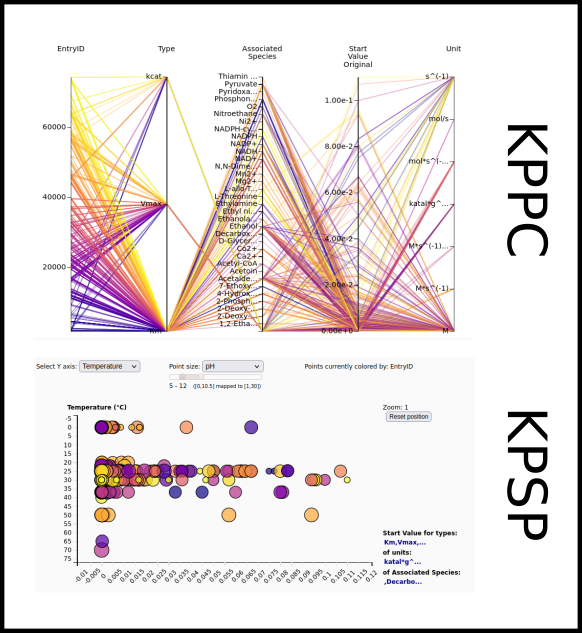
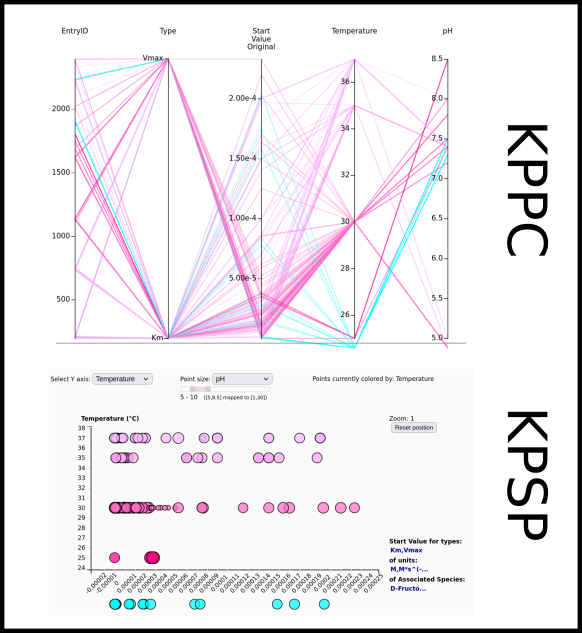
To improve SABIO-RK data interoperability, semantic markup was added to web pages as described and defined by
This structured information makes it easier to discover, collate and analyse our data.
|
51001
|
Phylogenetic analysis of ADP-glucose pyrophosphorylase subunits reveals a role of subunit interfaces in the ...
|
|
51002
|
Phylogenetic analysis of ADP-glucose pyrophosphorylase subunits reveals a role of subunit interfaces in the ...
|
|
51003
|
Phylogenetic analysis of ADP-glucose pyrophosphorylase subunits reveals a role of subunit interfaces in the ...
|
|
51004
|
Phylogenetic analysis of ADP-glucose pyrophosphorylase subunits reveals a role of subunit interfaces in the ...
|
|
51005
|
Phylogenetic analysis of ADP-glucose pyrophosphorylase subunits reveals a role of subunit interfaces in the ...
|
|
51006
|
Phylogenetic analysis of ADP-glucose pyrophosphorylase subunits reveals a role of subunit interfaces in the ...
|
|
51007
|
Phylogenetic analysis of ADP-glucose pyrophosphorylase subunits reveals a role of subunit interfaces in the ...
|
|
51008
|
Phylogenetic analysis of ADP-glucose pyrophosphorylase subunits reveals a role of subunit interfaces in the ...
|
|
51009
|
Phylogenetic analysis of ADP-glucose pyrophosphorylase subunits reveals a role of subunit interfaces in the ...
|
|
51010
|
Probing allosteric binding sites of the maize endosperm ADP-glucose pyrophosphorylase
|
|
51011
|
Probing allosteric binding sites of the maize endosperm ADP-glucose pyrophosphorylase
|
|
51012
|
Probing allosteric binding sites of the maize endosperm ADP-glucose pyrophosphorylase
|
|
51013
|
Probing allosteric binding sites of the maize endosperm ADP-glucose pyrophosphorylase
|
|
51014
|
Probing allosteric binding sites of the maize endosperm ADP-glucose pyrophosphorylase
|
|
51015
|
Probing allosteric binding sites of the maize endosperm ADP-glucose pyrophosphorylase
|
|
51016
|
Probing allosteric binding sites of the maize endosperm ADP-glucose pyrophosphorylase
|
|
51017
|
Probing allosteric binding sites of the maize endosperm ADP-glucose pyrophosphorylase
|
|
51018
|
Probing allosteric binding sites of the maize endosperm ADP-glucose pyrophosphorylase
|
|
51019
|
Probing allosteric binding sites of the maize endosperm ADP-glucose pyrophosphorylase
|
|
51020
|
Probing allosteric binding sites of the maize endosperm ADP-glucose pyrophosphorylase
|
|
51021
|
Probing allosteric binding sites of the maize endosperm ADP-glucose pyrophosphorylase
|
|
51022
|
Probing allosteric binding sites of the maize endosperm ADP-glucose pyrophosphorylase
|
|
51023
|
Probing allosteric binding sites of the maize endosperm ADP-glucose pyrophosphorylase
|
|
51024
|
Probing allosteric binding sites of the maize endosperm ADP-glucose pyrophosphorylase
|
|
51025
|
Probing allosteric binding sites of the maize endosperm ADP-glucose pyrophosphorylase
|
|
51026
|
Probing allosteric binding sites of the maize endosperm ADP-glucose pyrophosphorylase
|
|
51027
|
Probing allosteric binding sites of the maize endosperm ADP-glucose pyrophosphorylase
|
|
51028
|
Probing allosteric binding sites of the maize endosperm ADP-glucose pyrophosphorylase
|
|
51029
|
Probing allosteric binding sites of the maize endosperm ADP-glucose pyrophosphorylase
|
|
51030
|
Probing allosteric binding sites of the maize endosperm ADP-glucose pyrophosphorylase
|
|
51031
|
Probing allosteric binding sites of the maize endosperm ADP-glucose pyrophosphorylase
|
|
51032
|
Probing allosteric binding sites of the maize endosperm ADP-glucose pyrophosphorylase
|
|
51033
|
Probing allosteric binding sites of the maize endosperm ADP-glucose pyrophosphorylase
|
|
51034
|
Probing allosteric binding sites of the maize endosperm ADP-glucose pyrophosphorylase
|
|
51035
|
Probing allosteric binding sites of the maize endosperm ADP-glucose pyrophosphorylase
|
|
51036
|
Probing allosteric binding sites of the maize endosperm ADP-glucose pyrophosphorylase
|
|
51037
|
Probing allosteric binding sites of the maize endosperm ADP-glucose pyrophosphorylase
|
|
51038
|
Probing allosteric binding sites of the maize endosperm ADP-glucose pyrophosphorylase
|
|
51039
|
Probing allosteric binding sites of the maize endosperm ADP-glucose pyrophosphorylase
|
|
51040
|
Probing allosteric binding sites of the maize endosperm ADP-glucose pyrophosphorylase
|
|
51041
|
Probing allosteric binding sites of the maize endosperm ADP-glucose pyrophosphorylase
|
|
51042
|
Probing allosteric binding sites of the maize endosperm ADP-glucose pyrophosphorylase
|
|
51043
|
Probing allosteric binding sites of the maize endosperm ADP-glucose pyrophosphorylase
|
|
51044
|
Probing allosteric binding sites of the maize endosperm ADP-glucose pyrophosphorylase
|
|
51045
|
Probing allosteric binding sites of the maize endosperm ADP-glucose pyrophosphorylase
|
|
51046
|
Probing allosteric binding sites of the maize endosperm ADP-glucose pyrophosphorylase
|
|
51047
|
Probing allosteric binding sites of the maize endosperm ADP-glucose pyrophosphorylase
|
|
51048
|
Probing allosteric binding sites of the maize endosperm ADP-glucose pyrophosphorylase
|
|
51049
|
Probing allosteric binding sites of the maize endosperm ADP-glucose pyrophosphorylase
|
|
51050
|
Probing allosteric binding sites of the maize endosperm ADP-glucose pyrophosphorylase
|
|
51051
|
Probing allosteric binding sites of the maize endosperm ADP-glucose pyrophosphorylase
|
|
51052
|
Probing allosteric binding sites of the maize endosperm ADP-glucose pyrophosphorylase
|
|
51053
|
Probing allosteric binding sites of the maize endosperm ADP-glucose pyrophosphorylase
|
|
51054
|
Probing allosteric binding sites of the maize endosperm ADP-glucose pyrophosphorylase
|
|
51055
|
Probing allosteric binding sites of the maize endosperm ADP-glucose pyrophosphorylase
|
|
51056
|
Probing allosteric binding sites of the maize endosperm ADP-glucose pyrophosphorylase
|
|
51057
|
Probing allosteric binding sites of the maize endosperm ADP-glucose pyrophosphorylase
|
|
51058
|
Probing allosteric binding sites of the maize endosperm ADP-glucose pyrophosphorylase
|
|
51059
|
Probing allosteric binding sites of the maize endosperm ADP-glucose pyrophosphorylase
|
|
51060
|
Probing allosteric binding sites of the maize endosperm ADP-glucose pyrophosphorylase
|
|
51061
|
Probing allosteric binding sites of the maize endosperm ADP-glucose pyrophosphorylase
|
|
51062
|
Probing allosteric binding sites of the maize endosperm ADP-glucose pyrophosphorylase
|
|
51063
|
Probing allosteric binding sites of the maize endosperm ADP-glucose pyrophosphorylase
|
|
51064
|
Probing allosteric binding sites of the maize endosperm ADP-glucose pyrophosphorylase
|
|
51065
|
Probing allosteric binding sites of the maize endosperm ADP-glucose pyrophosphorylase
|
|
51066
|
Probing allosteric binding sites of the maize endosperm ADP-glucose pyrophosphorylase
|
|
51067
|
Probing allosteric binding sites of the maize endosperm ADP-glucose pyrophosphorylase
|
|
51068
|
Probing allosteric binding sites of the maize endosperm ADP-glucose pyrophosphorylase
|
|
51069
|
Probing allosteric binding sites of the maize endosperm ADP-glucose pyrophosphorylase
|
|
51070
|
Probing allosteric binding sites of the maize endosperm ADP-glucose pyrophosphorylase
|
|
51071
|
Probing allosteric binding sites of the maize endosperm ADP-glucose pyrophosphorylase
|
|
51072
|
Probing allosteric binding sites of the maize endosperm ADP-glucose pyrophosphorylase
|
|
51073
|
Nitric-oxide dioxygenase activity and function of flavohemoglobins. sensitivity to nitric oxide and carbon ...
|
|
51074
|
Nitric-oxide dioxygenase activity and function of flavohemoglobins. sensitivity to nitric oxide and carbon ...
|
|
51075
|
Nitric-oxide dioxygenase activity and function of flavohemoglobins. sensitivity to nitric oxide and carbon ...
|
|
51076
|
Nitric-oxide dioxygenase activity and function of flavohemoglobins. sensitivity to nitric oxide and carbon ...
|
|
51077
|
Nitric-oxide dioxygenase activity and function of flavohemoglobins. sensitivity to nitric oxide and carbon ...
|
|
51078
|
Nitric-oxide dioxygenase activity and function of flavohemoglobins. sensitivity to nitric oxide and carbon ...
|
|
51079
|
Nitric-oxide dioxygenase activity and function of flavohemoglobins. sensitivity to nitric oxide and carbon ...
|
|
51080
|
Nitric-oxide dioxygenase activity and function of flavohemoglobins. sensitivity to nitric oxide and carbon ...
|
|
51081
|
Nitric-oxide dioxygenase activity and function of flavohemoglobins. sensitivity to nitric oxide and carbon ...
|
|
51082
|
Nitric-oxide dioxygenase activity and function of flavohemoglobins. sensitivity to nitric oxide and carbon ...
|
|
51083
|
Nitric-oxide dioxygenase activity and function of flavohemoglobins. sensitivity to nitric oxide and carbon ...
|
|
51084
|
Nitric-oxide dioxygenase activity and function of flavohemoglobins. sensitivity to nitric oxide and carbon ...
|
|
51085
|
Nitric-oxide dioxygenase activity and function of flavohemoglobins. sensitivity to nitric oxide and carbon ...
|
|
51086
|
Nitric-oxide dioxygenase activity and function of flavohemoglobins. sensitivity to nitric oxide and carbon ...
|
|
51087
|
Nitric-oxide dioxygenase activity and function of flavohemoglobins. sensitivity to nitric oxide and carbon ...
|
|
51088
|
Nitric-oxide dioxygenase activity and function of flavohemoglobins. sensitivity to nitric oxide and carbon ...
|
|
51089
|
Nitric-oxide dioxygenase activity and function of flavohemoglobins. sensitivity to nitric oxide and carbon ...
|
|
51090
|
Nitric-oxide dioxygenase activity and function of flavohemoglobins. sensitivity to nitric oxide and carbon ...
|
|
51091
|
Nitric-oxide dioxygenase activity and function of flavohemoglobins. sensitivity to nitric oxide and carbon ...
|
|
51092
|
Nitric-oxide dioxygenase activity and function of flavohemoglobins. sensitivity to nitric oxide and carbon ...
|
|
51093
|
Nitric-oxide dioxygenase activity and function of flavohemoglobins. sensitivity to nitric oxide and carbon ...
|
|
51094
|
Nitric-oxide dioxygenase activity and function of flavohemoglobins. sensitivity to nitric oxide and carbon ...
|
|
51095
|
Nitric-oxide dioxygenase activity and function of flavohemoglobins. sensitivity to nitric oxide and carbon ...
|
|
51096
|
Nitric-oxide dioxygenase activity and function of flavohemoglobins. sensitivity to nitric oxide and carbon ...
|
|
51097
|
Nitric-oxide dioxygenase activity and function of flavohemoglobins. sensitivity to nitric oxide and carbon ...
|
|
51098
|
Nitric-oxide dioxygenase activity and function of flavohemoglobins. sensitivity to nitric oxide and carbon ...
|
|
51099
|
Nitric-oxide dioxygenase activity and function of flavohemoglobins. sensitivity to nitric oxide and carbon ...
|
|
51100
|
Nitric-oxide dioxygenase activity and function of flavohemoglobins. sensitivity to nitric oxide and carbon ...
|
|
51101
|
Nitric-oxide dioxygenase activity and function of flavohemoglobins. sensitivity to nitric oxide and carbon ...
|
|
51102
|
Nitric-oxide dioxygenase activity and function of flavohemoglobins. sensitivity to nitric oxide and carbon ...
|
|
51103
|
Nitric-oxide dioxygenase activity and function of flavohemoglobins. sensitivity to nitric oxide and carbon ...
|
|
51104
|
Nitric-oxide dioxygenase activity and function of flavohemoglobins. sensitivity to nitric oxide and carbon ...
|
|
51105
|
Nitric-oxide dioxygenase activity and function of flavohemoglobins. sensitivity to nitric oxide and carbon ...
|
|
51106
|
Design and synthesis of substrate and intermediate analogue inhibitors of S-ribosylhomocysteinase
|
|
51107
|
Design and synthesis of substrate and intermediate analogue inhibitors of S-ribosylhomocysteinase
|
|
51108
|
Design and synthesis of substrate and intermediate analogue inhibitors of S-ribosylhomocysteinase
|
|
51109
|
Design and synthesis of substrate and intermediate analogue inhibitors of S-ribosylhomocysteinase
|
|
51110
|
Design and synthesis of substrate and intermediate analogue inhibitors of S-ribosylhomocysteinase
|
|
51111
|
Design and synthesis of substrate and intermediate analogue inhibitors of S-ribosylhomocysteinase
|
|
51112
|
Design and synthesis of substrate and intermediate analogue inhibitors of S-ribosylhomocysteinase
|
|
51113
|
Design and synthesis of substrate and intermediate analogue inhibitors of S-ribosylhomocysteinase
|
|
51114
|
Design and synthesis of substrate and intermediate analogue inhibitors of S-ribosylhomocysteinase
|
|
51115
|
Design and synthesis of substrate and intermediate analogue inhibitors of S-ribosylhomocysteinase
|
|
51116
|
Design and synthesis of substrate and intermediate analogue inhibitors of S-ribosylhomocysteinase
|
|
51117
|
Design and synthesis of substrate and intermediate analogue inhibitors of S-ribosylhomocysteinase
|
|
51118
|
Design and synthesis of substrate and intermediate analogue inhibitors of S-ribosylhomocysteinase
|
|
51119
|
Design and synthesis of substrate and intermediate analogue inhibitors of S-ribosylhomocysteinase
|
|
51120
|
Design and synthesis of substrate and intermediate analogue inhibitors of S-ribosylhomocysteinase
|
|
51121
|
Design and synthesis of substrate and intermediate analogue inhibitors of S-ribosylhomocysteinase
|
|
51122
|
Design and synthesis of substrate and intermediate analogue inhibitors of S-ribosylhomocysteinase
|
|
51123
|
Design and synthesis of substrate and intermediate analogue inhibitors of S-ribosylhomocysteinase
|
|
51124
|
Design and synthesis of substrate and intermediate analogue inhibitors of S-ribosylhomocysteinase
|
|
51125
|
Design and synthesis of substrate and intermediate analogue inhibitors of S-ribosylhomocysteinase
|
|
51126
|
Design and synthesis of substrate and intermediate analogue inhibitors of S-ribosylhomocysteinase
|
|
51127
|
Coexisting kinetically distinguishable forms of dialkylglycine decarboxylase engendered by alkali metal ions
|
|
51128
|
Coexisting kinetically distinguishable forms of dialkylglycine decarboxylase engendered by alkali metal ions
|
|
51129
|
Coexisting kinetically distinguishable forms of dialkylglycine decarboxylase engendered by alkali metal ions
|
|
51130
|
Coexisting kinetically distinguishable forms of dialkylglycine decarboxylase engendered by alkali metal ions
|
|
51131
|
Kinetic, spectroscopic, and X-ray crystallographic characterization of the functional E151H aminopeptidase ...
|
|
51132
|
Kinetic, spectroscopic, and X-ray crystallographic characterization of the functional E151H aminopeptidase ...
|
|
51133
|
Kinetic, spectroscopic, and X-ray crystallographic characterization of the functional E151H aminopeptidase ...
|
|
51134
|
Kinetic, spectroscopic, and X-ray crystallographic characterization of the functional E151H aminopeptidase ...
|
|
51135
|
Two cinnamoyl-CoA reductase (CCR) genes from Arabidopsis thaliana are differentially expressed during ...
|
|
51136
|
Two cinnamoyl-CoA reductase (CCR) genes from Arabidopsis thaliana are differentially expressed during ...
|
|
51137
|
Two cinnamoyl-CoA reductase (CCR) genes from Arabidopsis thaliana are differentially expressed during ...
|
|
51138
|
Two cinnamoyl-CoA reductase (CCR) genes from Arabidopsis thaliana are differentially expressed during ...
|
|
51139
|
Two cinnamoyl-CoA reductase (CCR) genes from Arabidopsis thaliana are differentially expressed during ...
|
|
51140
|
Two cinnamoyl-CoA reductase (CCR) genes from Arabidopsis thaliana are differentially expressed during ...
|
|
51141
|
Kinetic and inhibition studies of cinnamoyl-CoA reductase 1 from Arabidopsis thaliana.
|
|
51142
|
Kinetic and inhibition studies of cinnamoyl-CoA reductase 1 from Arabidopsis thaliana.
|
|
51143
|
Kinetic and inhibition studies of cinnamoyl-CoA reductase 1 from Arabidopsis thaliana.
|
|
51144
|
Kinetic and inhibition studies of cinnamoyl-CoA reductase 1 from Arabidopsis thaliana.
|
|
51145
|
Kinetic and inhibition studies of cinnamoyl-CoA reductase 1 from Arabidopsis thaliana.
|
|
51146
|
Kinetic and inhibition studies of cinnamoyl-CoA reductase 1 from Arabidopsis thaliana.
|
|
51147
|
Kinetic and inhibition studies of cinnamoyl-CoA reductase 1 from Arabidopsis thaliana.
|
|
51148
|
Kinetic and inhibition studies of cinnamoyl-CoA reductase 1 from Arabidopsis thaliana.
|
|
51149
|
Kinetic and inhibition studies of cinnamoyl-CoA reductase 1 from Arabidopsis thaliana.
|
|
51150
|
Kinetic and inhibition studies of cinnamoyl-CoA reductase 1 from Arabidopsis thaliana.
|
|
51151
|
Kinetic and inhibition studies of cinnamoyl-CoA reductase 1 from Arabidopsis thaliana.
|
|
51152
|
Kinetic and inhibition studies of cinnamoyl-CoA reductase 1 from Arabidopsis thaliana.
|
|
51153
|
Kinetic and inhibition studies of cinnamoyl-CoA reductase 1 from Arabidopsis thaliana.
|
|
51154
|
Kinetic and inhibition studies of cinnamoyl-CoA reductase 1 from Arabidopsis thaliana.
|
|
51155
|
Kinetic and inhibition studies of cinnamoyl-CoA reductase 1 from Arabidopsis thaliana.
|
|
51156
|
Kinetic and inhibition studies of cinnamoyl-CoA reductase 1 from Arabidopsis thaliana.
|
|
51157
|
His-391 of beta-galactosidase (Escherichia coli) promotes catalyses by strong interactions with the transition ...
|
|
51158
|
His-391 of beta-galactosidase (Escherichia coli) promotes catalyses by strong interactions with the transition ...
|
|
51159
|
His-391 of beta-galactosidase (Escherichia coli) promotes catalyses by strong interactions with the transition ...
|
|
51160
|
His-391 of beta-galactosidase (Escherichia coli) promotes catalyses by strong interactions with the transition ...
|
|
51161
|
His-391 of beta-galactosidase (Escherichia coli) promotes catalyses by strong interactions with the transition ...
|
|
51162
|
His-391 of beta-galactosidase (Escherichia coli) promotes catalyses by strong interactions with the transition ...
|
|
51163
|
His-391 of beta-galactosidase (Escherichia coli) promotes catalyses by strong interactions with the transition ...
|
|
51164
|
His-391 of beta-galactosidase (Escherichia coli) promotes catalyses by strong interactions with the transition ...
|
|
51165
|
His-391 of beta-galactosidase (Escherichia coli) promotes catalyses by strong interactions with the transition ...
|
|
51166
|
His-391 of beta-galactosidase (Escherichia coli) promotes catalyses by strong interactions with the transition ...
|
|
51167
|
His-391 of beta-galactosidase (Escherichia coli) promotes catalyses by strong interactions with the transition ...
|
|
51168
|
His-391 of beta-galactosidase (Escherichia coli) promotes catalyses by strong interactions with the transition ...
|
|
51169
|
His-391 of beta-galactosidase (Escherichia coli) promotes catalyses by strong interactions with the transition ...
|
|
51170
|
His-391 of beta-galactosidase (Escherichia coli) promotes catalyses by strong interactions with the transition ...
|
|
51171
|
His-391 of beta-galactosidase (Escherichia coli) promotes catalyses by strong interactions with the transition ...
|
|
51172
|
His-391 of beta-galactosidase (Escherichia coli) promotes catalyses by strong interactions with the transition ...
|
|
51173
|
His-391 of beta-galactosidase (Escherichia coli) promotes catalyses by strong interactions with the transition ...
|
|
51174
|
His-391 of beta-galactosidase (Escherichia coli) promotes catalyses by strong interactions with the transition ...
|
|
51175
|
His-391 of beta-galactosidase (Escherichia coli) promotes catalyses by strong interactions with the transition ...
|
|
51176
|
His-391 of beta-galactosidase (Escherichia coli) promotes catalyses by strong interactions with the transition ...
|
|
51177
|
His-391 of beta-galactosidase (Escherichia coli) promotes catalyses by strong interactions with the transition ...
|
|
51178
|
His-391 of beta-galactosidase (Escherichia coli) promotes catalyses by strong interactions with the transition ...
|
|
51179
|
His-391 of beta-galactosidase (Escherichia coli) promotes catalyses by strong interactions with the transition ...
|
|
51180
|
His-391 of beta-galactosidase (Escherichia coli) promotes catalyses by strong interactions with the transition ...
|
|
51181
|
His-391 of beta-galactosidase (Escherichia coli) promotes catalyses by strong interactions with the transition ...
|
|
51182
|
His-391 of beta-galactosidase (Escherichia coli) promotes catalyses by strong interactions with the transition ...
|
|
51183
|
His-391 of beta-galactosidase (Escherichia coli) promotes catalyses by strong interactions with the transition ...
|
|
51184
|
His-391 of beta-galactosidase (Escherichia coli) promotes catalyses by strong interactions with the transition ...
|
|
51185
|
His-391 of beta-galactosidase (Escherichia coli) promotes catalyses by strong interactions with the transition ...
|
|
51186
|
His-391 of beta-galactosidase (Escherichia coli) promotes catalyses by strong interactions with the transition ...
|
|
51187
|
His-391 of beta-galactosidase (Escherichia coli) promotes catalyses by strong interactions with the transition ...
|
|
51188
|
His-391 of beta-galactosidase (Escherichia coli) promotes catalyses by strong interactions with the transition ...
|
|
51189
|
His-391 of beta-galactosidase (Escherichia coli) promotes catalyses by strong interactions with the transition ...
|
|
51190
|
His-391 of beta-galactosidase (Escherichia coli) promotes catalyses by strong interactions with the transition ...
|
|
51191
|
His-391 of beta-galactosidase (Escherichia coli) promotes catalyses by strong interactions with the transition ...
|
|
51192
|
His-391 of beta-galactosidase (Escherichia coli) promotes catalyses by strong interactions with the transition ...
|
|
51193
|
His-391 of beta-galactosidase (Escherichia coli) promotes catalyses by strong interactions with the transition ...
|
|
51194
|
His-391 of beta-galactosidase (Escherichia coli) promotes catalyses by strong interactions with the transition ...
|
|
51195
|
His-391 of beta-galactosidase (Escherichia coli) promotes catalyses by strong interactions with the transition ...
|
|
51196
|
His-391 of beta-galactosidase (Escherichia coli) promotes catalyses by strong interactions with the transition ...
|
|
51197
|
His-391 of beta-galactosidase (Escherichia coli) promotes catalyses by strong interactions with the transition ...
|
|
51198
|
His-391 of beta-galactosidase (Escherichia coli) promotes catalyses by strong interactions with the transition ...
|
|
51199
|
His-391 of beta-galactosidase (Escherichia coli) promotes catalyses by strong interactions with the transition ...
|
|
51200
|
His-391 of beta-galactosidase (Escherichia coli) promotes catalyses by strong interactions with the transition ...
|
|
51201
|
His-391 of beta-galactosidase (Escherichia coli) promotes catalyses by strong interactions with the transition ...
|
|
51202
|
His-391 of beta-galactosidase (Escherichia coli) promotes catalyses by strong interactions with the transition ...
|
|
51203
|
His-391 of beta-galactosidase (Escherichia coli) promotes catalyses by strong interactions with the transition ...
|
|
51204
|
His-391 of beta-galactosidase (Escherichia coli) promotes catalyses by strong interactions with the transition ...
|
|
51205
|
His-391 of beta-galactosidase (Escherichia coli) promotes catalyses by strong interactions with the transition ...
|
|
51206
|
His-391 of beta-galactosidase (Escherichia coli) promotes catalyses by strong interactions with the transition ...
|
|
51207
|
His-391 of beta-galactosidase (Escherichia coli) promotes catalyses by strong interactions with the transition ...
|
|
51208
|
His-391 of beta-galactosidase (Escherichia coli) promotes catalyses by strong interactions with the transition ...
|
|
51209
|
His-391 of beta-galactosidase (Escherichia coli) promotes catalyses by strong interactions with the transition ...
|
|
51210
|
His-391 of beta-galactosidase (Escherichia coli) promotes catalyses by strong interactions with the transition ...
|
|
51211
|
His-391 of beta-galactosidase (Escherichia coli) promotes catalyses by strong interactions with the transition ...
|
|
51212
|
His-391 of beta-galactosidase (Escherichia coli) promotes catalyses by strong interactions with the transition ...
|
|
51213
|
His-391 of beta-galactosidase (Escherichia coli) promotes catalyses by strong interactions with the transition ...
|
|
51214
|
His-391 of beta-galactosidase (Escherichia coli) promotes catalyses by strong interactions with the transition ...
|
|
51215
|
His-391 of beta-galactosidase (Escherichia coli) promotes catalyses by strong interactions with the transition ...
|
|
51216
|
His-391 of beta-galactosidase (Escherichia coli) promotes catalyses by strong interactions with the transition ...
|
|
51217
|
His-391 of beta-galactosidase (Escherichia coli) promotes catalyses by strong interactions with the transition ...
|
|
51218
|
His-391 of beta-galactosidase (Escherichia coli) promotes catalyses by strong interactions with the transition ...
|
|
51219
|
His-391 of beta-galactosidase (Escherichia coli) promotes catalyses by strong interactions with the transition ...
|
|
51220
|
His-391 of beta-galactosidase (Escherichia coli) promotes catalyses by strong interactions with the transition ...
|
|
51221
|
His-391 of beta-galactosidase (Escherichia coli) promotes catalyses by strong interactions with the transition ...
|
|
51222
|
His-391 of beta-galactosidase (Escherichia coli) promotes catalyses by strong interactions with the transition ...
|
|
51223
|
His-391 of beta-galactosidase (Escherichia coli) promotes catalyses by strong interactions with the transition ...
|
|
51224
|
His-391 of beta-galactosidase (Escherichia coli) promotes catalyses by strong interactions with the transition ...
|
|
51225
|
His-391 of beta-galactosidase (Escherichia coli) promotes catalyses by strong interactions with the transition ...
|
|
51226
|
His-391 of beta-galactosidase (Escherichia coli) promotes catalyses by strong interactions with the transition ...
|
|
51227
|
His-391 of beta-galactosidase (Escherichia coli) promotes catalyses by strong interactions with the transition ...
|
|
51228
|
His-391 of beta-galactosidase (Escherichia coli) promotes catalyses by strong interactions with the transition ...
|
|
51229
|
His-391 of beta-galactosidase (Escherichia coli) promotes catalyses by strong interactions with the transition ...
|
|
51230
|
His-391 of beta-galactosidase (Escherichia coli) promotes catalyses by strong interactions with the transition ...
|
|
51231
|
His-391 of beta-galactosidase (Escherichia coli) promotes catalyses by strong interactions with the transition ...
|
|
51232
|
His-391 of beta-galactosidase (Escherichia coli) promotes catalyses by strong interactions with the transition ...
|
|
51233
|
His-391 of beta-galactosidase (Escherichia coli) promotes catalyses by strong interactions with the transition ...
|
|
51234
|
His-391 of beta-galactosidase (Escherichia coli) promotes catalyses by strong interactions with the transition ...
|
|
51235
|
His-391 of beta-galactosidase (Escherichia coli) promotes catalyses by strong interactions with the transition ...
|
|
51236
|
His-391 of beta-galactosidase (Escherichia coli) promotes catalyses by strong interactions with the transition ...
|
|
51237
|
His-391 of beta-galactosidase (Escherichia coli) promotes catalyses by strong interactions with the transition ...
|
|
51238
|
His-391 of beta-galactosidase (Escherichia coli) promotes catalyses by strong interactions with the transition ...
|
|
51239
|
His-391 of beta-galactosidase (Escherichia coli) promotes catalyses by strong interactions with the transition ...
|
|
51240
|
His-391 of beta-galactosidase (Escherichia coli) promotes catalyses by strong interactions with the transition ...
|
|
51241
|
His-391 of beta-galactosidase (Escherichia coli) promotes catalyses by strong interactions with the transition ...
|
|
51242
|
His-391 of beta-galactosidase (Escherichia coli) promotes catalyses by strong interactions with the transition ...
|
|
51243
|
His-391 of beta-galactosidase (Escherichia coli) promotes catalyses by strong interactions with the transition ...
|
|
51244
|
His-391 of beta-galactosidase (Escherichia coli) promotes catalyses by strong interactions with the transition ...
|
|
51245
|
Intermediate instability at high temperature leads to low pathway efficiency for an in vitro reconstituted ...
|
|
51246
|
Intermediate instability at high temperature leads to low pathway efficiency for an in vitro reconstituted ...
|
|
51247
|
Intermediate instability at high temperature leads to low pathway efficiency for an in vitro reconstituted ...
|
|
51248
|
Intermediate instability at high temperature leads to low pathway efficiency for an in vitro reconstituted ...
|
|
51249
|
alpha-ketoglutaric semialdehyde dehydrogenase isozymes involved in metabolic pathways of D-glucarate, ...
|
|
51250
|
alpha-ketoglutaric semialdehyde dehydrogenase isozymes involved in metabolic pathways of D-glucarate, ...
|
|
51251
|
alpha-ketoglutaric semialdehyde dehydrogenase isozymes involved in metabolic pathways of D-glucarate, ...
|
|
51252
|
alpha-ketoglutaric semialdehyde dehydrogenase isozymes involved in metabolic pathways of D-glucarate, ...
|
|
51253
|
alpha-ketoglutaric semialdehyde dehydrogenase isozymes involved in metabolic pathways of D-glucarate, ...
|
|
51254
|
alpha-ketoglutaric semialdehyde dehydrogenase isozymes involved in metabolic pathways of D-glucarate, ...
|
|
51255
|
alpha-ketoglutaric semialdehyde dehydrogenase isozymes involved in metabolic pathways of D-glucarate, ...
|
|
51256
|
alpha-ketoglutaric semialdehyde dehydrogenase isozymes involved in metabolic pathways of D-glucarate, ...
|
|
51257
|
alpha-ketoglutaric semialdehyde dehydrogenase isozymes involved in metabolic pathways of D-glucarate, ...
|
|
51258
|
alpha-ketoglutaric semialdehyde dehydrogenase isozymes involved in metabolic pathways of D-glucarate, ...
|
|
51259
|
alpha-ketoglutaric semialdehyde dehydrogenase isozymes involved in metabolic pathways of D-glucarate, ...
|
|
51260
|
alpha-ketoglutaric semialdehyde dehydrogenase isozymes involved in metabolic pathways of D-glucarate, ...
|
|
51261
|
alpha-ketoglutaric semialdehyde dehydrogenase isozymes involved in metabolic pathways of D-glucarate, ...
|
|
51262
|
alpha-ketoglutaric semialdehyde dehydrogenase isozymes involved in metabolic pathways of D-glucarate, ...
|
|
51263
|
alpha-ketoglutaric semialdehyde dehydrogenase isozymes involved in metabolic pathways of D-glucarate, ...
|
|
51264
|
alpha-ketoglutaric semialdehyde dehydrogenase isozymes involved in metabolic pathways of D-glucarate, ...
|
|
51265
|
alpha-ketoglutaric semialdehyde dehydrogenase isozymes involved in metabolic pathways of D-glucarate, ...
|
|
51266
|
alpha-ketoglutaric semialdehyde dehydrogenase isozymes involved in metabolic pathways of D-glucarate, ...
|
|
51267
|
alpha-ketoglutaric semialdehyde dehydrogenase isozymes involved in metabolic pathways of D-glucarate, ...
|
|
51268
|
alpha-ketoglutaric semialdehyde dehydrogenase isozymes involved in metabolic pathways of D-glucarate, ...
|
|
51269
|
Purification and characterization of a recombinant alpha-N-acetylgalactosaminidase from Clostridium ...
|
|
51270
|
Purification and characterization of a recombinant alpha-N-acetylgalactosaminidase from Clostridium ...
|
|
51271
|
A novel hyperthermophilic archaeal glyoxylate reductase from Thermococcus litoralis. Characterization, gene ...
|
|
51272
|
A novel hyperthermophilic archaeal glyoxylate reductase from Thermococcus litoralis. Characterization, gene ...
|
|
51273
|
A novel hyperthermophilic archaeal glyoxylate reductase from Thermococcus litoralis. Characterization, gene ...
|
|
51274
|
A novel hyperthermophilic archaeal glyoxylate reductase from Thermococcus litoralis. Characterization, gene ...
|
|
51275
|
Sialidase, receptor-binding and fusion-promotion activities of Newcastle disease virus ...
|
|
51276
|
Sialidase, receptor-binding and fusion-promotion activities of Newcastle disease virus ...
|
|
51277
|
Sialidase, receptor-binding and fusion-promotion activities of Newcastle disease virus ...
|
|
51278
|
Unexpected Divergence of Enzyme Function and Sequence: "N-Acylamino Acid Racemase" Is o-Succinylbenzoate ...
|
|
51279
|
Unexpected Divergence of Enzyme Function and Sequence: "N-Acylamino Acid Racemase" Is o-Succinylbenzoate ...
|
|
51280
|
Unexpected Divergence of Enzyme Function and Sequence: "N-Acylamino Acid Racemase" Is o-Succinylbenzoate ...
|
|
51281
|
Unexpected Divergence of Enzyme Function and Sequence: "N-Acylamino Acid Racemase" Is o-Succinylbenzoate ...
|
|
51282
|
Unexpected Divergence of Enzyme Function and Sequence: "N-Acylamino Acid Racemase" Is o-Succinylbenzoate ...
|
|
51283
|
Unexpected Divergence of Enzyme Function and Sequence: "N-Acylamino Acid Racemase" Is o-Succinylbenzoate ...
|
|
51284
|
Unexpected Divergence of Enzyme Function and Sequence: "N-Acylamino Acid Racemase" Is o-Succinylbenzoate ...
|
|
51285
|
Unexpected Divergence of Enzyme Function and Sequence: "N-Acylamino Acid Racemase" Is o-Succinylbenzoate ...
|
|
51286
|
The identity of the active site of oxalate decarboxylase and the importance of the stability of active-site ...
|
|
51287
|
The identity of the active site of oxalate decarboxylase and the importance of the stability of active-site ...
|
|
51288
|
The identity of the active site of oxalate decarboxylase and the importance of the stability of active-site ...
|
|
51289
|
The identity of the active site of oxalate decarboxylase and the importance of the stability of active-site ...
|
|
51290
|
The identity of the active site of oxalate decarboxylase and the importance of the stability of active-site ...
|
|
51291
|
The identity of the active site of oxalate decarboxylase and the importance of the stability of active-site ...
|
|
51292
|
The identity of the active site of oxalate decarboxylase and the importance of the stability of active-site ...
|
|
51293
|
The identity of the active site of oxalate decarboxylase and the importance of the stability of active-site ...
|
|
51294
|
The identity of the active site of oxalate decarboxylase and the importance of the stability of active-site ...
|
|
51295
|
Evolutionary potential of (beta/alpha)8-barrels: in vitro enhancement of a "new" reaction in the enolase ...
|
|
51296
|
Evolutionary potential of (beta/alpha)8-barrels: in vitro enhancement of a "new" reaction in the enolase ...
|
|
51297
|
Evolutionary potential of (beta/alpha)8-barrels: in vitro enhancement of a "new" reaction in the enolase ...
|
|
51298
|
Evolutionary potential of (beta/alpha)8-barrels: in vitro enhancement of a "new" reaction in the enolase ...
|
|
51299
|
Evolutionary potential of (beta/alpha)8-barrels: in vitro enhancement of a "new" reaction in the enolase ...
|
|
51300
|
Evolutionary potential of (beta/alpha)8-barrels: in vitro enhancement of a "new" reaction in the enolase ...
|
|
51301
|
Evolutionary potential of (beta/alpha)8-barrels: in vitro enhancement of a "new" reaction in the enolase ...
|
|
51302
|
Evolutionary potential of (beta/alpha)8-barrels: in vitro enhancement of a "new" reaction in the enolase ...
|
|
51303
|
Evolutionary potential of (beta/alpha)8-barrels: in vitro enhancement of a "new" reaction in the enolase ...
|
|
51304
|
Evolutionary potential of (beta/alpha)8-barrels: in vitro enhancement of a "new" reaction in the enolase ...
|
|
51305
|
Evolutionary potential of (beta/alpha)8-barrels: in vitro enhancement of a "new" reaction in the enolase ...
|
|
51306
|
Evolutionary potential of (beta/alpha)8-barrels: in vitro enhancement of a "new" reaction in the enolase ...
|
|
51307
|
Evolutionary potential of (beta/alpha)8-barrels: in vitro enhancement of a "new" reaction in the enolase ...
|
|
51308
|
Phytochelatin synthase catalyzes key step in turnover of glutathione conjugates
|
|
51309
|
Phytochelatin synthase catalyzes key step in turnover of glutathione conjugates
|
|
51310
|
Phytochelatin synthase catalyzes key step in turnover of glutathione conjugates
|
|
51311
|
Phytochelatin synthase catalyzes key step in turnover of glutathione conjugates
|
|
51312
|
Phytochelatin synthase catalyzes key step in turnover of glutathione conjugates
|
|
51313
|
Structural and functional analysis of two glutamate racemase isozymes from Bacillus anthracis and implications ...
|
|
51314
|
Structural and functional analysis of two glutamate racemase isozymes from Bacillus anthracis and implications ...
|
|
51315
|
Structural and functional analysis of two glutamate racemase isozymes from Bacillus anthracis and implications ...
|
|
51316
|
Structural and functional analysis of two glutamate racemase isozymes from Bacillus anthracis and implications ...
|
|
51317
|
Structural and functional analysis of two glutamate racemase isozymes from Bacillus anthracis and implications ...
|
|
51318
|
Structural and functional analysis of two glutamate racemase isozymes from Bacillus anthracis and implications ...
|
|
51319
|
Structural and functional analysis of two glutamate racemase isozymes from Bacillus anthracis and implications ...
|
|
51320
|
Structural and functional analysis of two glutamate racemase isozymes from Bacillus anthracis and implications ...
|
|
51321
|
Structural and functional analysis of two glutamate racemase isozymes from Bacillus anthracis and implications ...
|
|
51322
|
Structural and functional analysis of two glutamate racemase isozymes from Bacillus anthracis and implications ...
|
|
51323
|
Structural and functional analysis of two glutamate racemase isozymes from Bacillus anthracis and implications ...
|
|
51324
|
Structural and functional analysis of two glutamate racemase isozymes from Bacillus anthracis and implications ...
|
|
51325
|
Structural and functional analysis of two glutamate racemase isozymes from Bacillus anthracis and implications ...
|
|
51326
|
Structural and functional analysis of two glutamate racemase isozymes from Bacillus anthracis and implications ...
|
|
51327
|
Structural and functional analysis of two glutamate racemase isozymes from Bacillus anthracis and implications ...
|
|
51328
|
Structural and functional analysis of two glutamate racemase isozymes from Bacillus anthracis and implications ...
|
|
51329
|
Structural and functional analysis of two glutamate racemase isozymes from Bacillus anthracis and implications ...
|
|
51330
|
GTPgammaS regulation of a 12-transmembrane guanylyl cyclase is retained after mutation to an adenylyl cyclase
|
|
51331
|
GTPgammaS regulation of a 12-transmembrane guanylyl cyclase is retained after mutation to an adenylyl cyclase
|
|
51332
|
GTPgammaS regulation of a 12-transmembrane guanylyl cyclase is retained after mutation to an adenylyl cyclase
|
|
51333
|
GTPgammaS regulation of a 12-transmembrane guanylyl cyclase is retained after mutation to an adenylyl cyclase
|
|
51334
|
GTPgammaS regulation of a 12-transmembrane guanylyl cyclase is retained after mutation to an adenylyl cyclase
|
|
51335
|
GTPgammaS regulation of a 12-transmembrane guanylyl cyclase is retained after mutation to an adenylyl cyclase
|
|
51336
|
Aminoacyl-coenzyme A synthesis catalyzed by adenylation domains
|
|
51337
|
Aminoacyl-coenzyme A synthesis catalyzed by adenylation domains
|
|
51338
|
Aminoacyl-coenzyme A synthesis catalyzed by adenylation domains
|
|
51339
|
Aminoacyl-coenzyme A synthesis catalyzed by adenylation domains
|
|
51340
|
Aminoacyl-coenzyme A synthesis catalyzed by adenylation domains
|
|
51341
|
Aminoacyl-coenzyme A synthesis catalyzed by adenylation domains
|
|
51342
|
Aminoacyl-coenzyme A synthesis catalyzed by adenylation domains
|
|
51343
|
Aminoacyl-coenzyme A synthesis catalyzed by adenylation domains
|
|
51344
|
Aminoacyl-coenzyme A synthesis catalyzed by adenylation domains
|
|
51345
|
Aminoacyl-coenzyme A synthesis catalyzed by adenylation domains
|
|
51346
|
Aminoacyl-coenzyme A synthesis catalyzed by adenylation domains
|
|
51347
|
Aminoacyl-coenzyme A synthesis catalyzed by adenylation domains
|
|
51348
|
Aminoacyl-coenzyme A synthesis catalyzed by adenylation domains
|
|
51349
|
Aminoacyl-coenzyme A synthesis catalyzed by adenylation domains
|
|
51350
|
Aminoacyl-coenzyme A synthesis catalyzed by adenylation domains
|
|
51351
|
Aminoacyl-coenzyme A synthesis catalyzed by adenylation domains
|
|
51352
|
Aminoacyl-coenzyme A synthesis catalyzed by adenylation domains
|
|
51353
|
Acyl coenzyme A thioesterase Them5/Acot15 is involved in cardiolipin remodeling and fatty liver development.
|
|
51354
|
Acyl coenzyme A thioesterase Them5/Acot15 is involved in cardiolipin remodeling and fatty liver development.
|
|
51355
|
Acyl coenzyme A thioesterase Them5/Acot15 is involved in cardiolipin remodeling and fatty liver development.
|
|
51356
|
Acyl coenzyme A thioesterase Them5/Acot15 is involved in cardiolipin remodeling and fatty liver development.
|
|
51357
|
Acyl coenzyme A thioesterase Them5/Acot15 is involved in cardiolipin remodeling and fatty liver development.
|
|
51358
|
Acyl coenzyme A thioesterase Them5/Acot15 is involved in cardiolipin remodeling and fatty liver development.
|
|
51359
|
Acyl coenzyme A thioesterase Them5/Acot15 is involved in cardiolipin remodeling and fatty liver development.
|
|
51360
|
Acyl coenzyme A thioesterase Them5/Acot15 is involved in cardiolipin remodeling and fatty liver development.
|
|
51361
|
Characterisation of a novel mouse liver aldo-keto reductase AKR7A5
|
|
51362
|
Characterisation of a novel mouse liver aldo-keto reductase AKR7A5
|
|
51363
|
Characterisation of a novel mouse liver aldo-keto reductase AKR7A5
|
|
51364
|
Characterisation of a novel mouse liver aldo-keto reductase AKR7A5
|
|
51365
|
Characterisation of a novel mouse liver aldo-keto reductase AKR7A5
|
|
51366
|
Characterisation of a novel mouse liver aldo-keto reductase AKR7A5
|
|
51367
|
Characterisation of a novel mouse liver aldo-keto reductase AKR7A5
|
|
51368
|
Characterisation of a novel mouse liver aldo-keto reductase AKR7A5
|
|
51369
|
Characterisation of a novel mouse liver aldo-keto reductase AKR7A5
|
|
51370
|
Characterisation of a novel mouse liver aldo-keto reductase AKR7A5
|
|
51371
|
Characterisation of a novel mouse liver aldo-keto reductase AKR7A5
|
|
51372
|
Characterisation of a novel mouse liver aldo-keto reductase AKR7A5
|
|
51373
|
Characterisation of a novel mouse liver aldo-keto reductase AKR7A5
|
|
51374
|
Characterisation of a novel mouse liver aldo-keto reductase AKR7A5
|
|
51375
|
Characterisation of a novel mouse liver aldo-keto reductase AKR7A5
|
|
51376
|
Characterisation of a novel mouse liver aldo-keto reductase AKR7A5
|
|
51377
|
Characterisation of a novel mouse liver aldo-keto reductase AKR7A5
|
|
51378
|
Characterisation of a novel mouse liver aldo-keto reductase AKR7A5
|
|
51379
|
Characterisation of a novel mouse liver aldo-keto reductase AKR7A5
|
|
51380
|
Characterisation of a novel mouse liver aldo-keto reductase AKR7A5
|
|
51381
|
Characterisation of a novel mouse liver aldo-keto reductase AKR7A5
|
|
51382
|
Characterisation of a novel mouse liver aldo-keto reductase AKR7A5
|
|
51383
|
Characterisation of a novel mouse liver aldo-keto reductase AKR7A5
|
|
51384
|
Kinetic and molecular properties of Bacillus subtilis IBTC-3 subtilisin
|
|
51385
|
Kinetic and molecular properties of Bacillus subtilis IBTC-3 subtilisin
|
|
51386
|
Kinetic and molecular properties of Bacillus subtilis IBTC-3 subtilisin
|
|
51387
|
Kinetic and molecular properties of Bacillus subtilis IBTC-3 subtilisin
|
|
51388
|
Kinetic and molecular properties of Bacillus subtilis IBTC-3 subtilisin
|
|
51389
|
Kinetic and molecular properties of Bacillus subtilis IBTC-3 subtilisin
|
|
51390
|
Kinetic and molecular properties of Bacillus subtilis IBTC-3 subtilisin
|
|
51391
|
Kinetic and molecular properties of Bacillus subtilis IBTC-3 subtilisin
|
|
51392
|
Kinetic and molecular properties of Bacillus subtilis IBTC-3 subtilisin
|
|
51393
|
Kinetic and molecular properties of Bacillus subtilis IBTC-3 subtilisin
|
|
51394
|
Kinetic and molecular properties of Bacillus subtilis IBTC-3 subtilisin
|
|
51395
|
Kinetic and molecular properties of Bacillus subtilis IBTC-3 subtilisin
|
|
51396
|
Kinetic and molecular properties of Bacillus subtilis IBTC-3 subtilisin
|
|
51397
|
The missing step of the L-galactose pathway of ascorbate biosynthesis in plants, an L-galactose ...
|
|
51398
|
The missing step of the L-galactose pathway of ascorbate biosynthesis in plants, an L-galactose ...
|
|
51399
|
The missing step of the L-galactose pathway of ascorbate biosynthesis in plants, an L-galactose ...
|
|
51400
|
X-ray, NMR, and mutational studies of the catalytic cycle of the GDP-mannose mannosyl hydrolase reaction
|
|
51401
|
X-ray, NMR, and mutational studies of the catalytic cycle of the GDP-mannose mannosyl hydrolase reaction
|
|
51402
|
Biochemical and molecular characterization of taurine:pyruvate aminotransferase from the anaerobe Bilophila ...
|
|
51403
|
Biochemical and molecular characterization of taurine:pyruvate aminotransferase from the anaerobe Bilophila ...
|
|
51404
|
Biochemical and molecular characterization of taurine:pyruvate aminotransferase from the anaerobe Bilophila ...
|
|
51405
|
Human MTH3 (NUDT18) protein hydrolyzes oxidized forms of guanosine and deoxyguanosine diphosphates: comparison ...
|
|
51406
|
Human MTH3 (NUDT18) protein hydrolyzes oxidized forms of guanosine and deoxyguanosine diphosphates: comparison ...
|
|
51407
|
Cytokinin-deficient transgenic Arabidopsis plants show multiple developmental alterations indicating opposite ...
|
|
51408
|
Cytokinin-deficient transgenic Arabidopsis plants show multiple developmental alterations indicating opposite ...
|
|
51409
|
Cytokinin-deficient transgenic Arabidopsis plants show multiple developmental alterations indicating opposite ...
|
|
51410
|
Cytokinin-deficient transgenic Arabidopsis plants show multiple developmental alterations indicating opposite ...
|
|
51411
|
Formamide probes a role for water in the catalytic cycle of cytochrome c oxidase
|
|
51412
|
Formamide probes a role for water in the catalytic cycle of cytochrome c oxidase
|
|
51413
|
His-357 of beta-galactosidase (Escherichia coli) interacts with the C3 hydroxyl in the transition state and ...
|
|
51414
|
His-357 of beta-galactosidase (Escherichia coli) interacts with the C3 hydroxyl in the transition state and ...
|
|
51415
|
His-357 of beta-galactosidase (Escherichia coli) interacts with the C3 hydroxyl in the transition state and ...
|
|
51416
|
His-357 of beta-galactosidase (Escherichia coli) interacts with the C3 hydroxyl in the transition state and ...
|
|
51417
|
His-357 of beta-galactosidase (Escherichia coli) interacts with the C3 hydroxyl in the transition state and ...
|
|
51418
|
His-357 of beta-galactosidase (Escherichia coli) interacts with the C3 hydroxyl in the transition state and ...
|
|
51419
|
His-357 of beta-galactosidase (Escherichia coli) interacts with the C3 hydroxyl in the transition state and ...
|
|
51420
|
His-357 of beta-galactosidase (Escherichia coli) interacts with the C3 hydroxyl in the transition state and ...
|
|
51421
|
His-357 of beta-galactosidase (Escherichia coli) interacts with the C3 hydroxyl in the transition state and ...
|
|
51422
|
His-357 of beta-galactosidase (Escherichia coli) interacts with the C3 hydroxyl in the transition state and ...
|
|
51423
|
His-357 of beta-galactosidase (Escherichia coli) interacts with the C3 hydroxyl in the transition state and ...
|
|
51424
|
His-357 of beta-galactosidase (Escherichia coli) interacts with the C3 hydroxyl in the transition state and ...
|
|
51425
|
His-357 of beta-galactosidase (Escherichia coli) interacts with the C3 hydroxyl in the transition state and ...
|
|
51426
|
His-357 of beta-galactosidase (Escherichia coli) interacts with the C3 hydroxyl in the transition state and ...
|
|
51427
|
His-357 of beta-galactosidase (Escherichia coli) interacts with the C3 hydroxyl in the transition state and ...
|
|
51428
|
His-357 of beta-galactosidase (Escherichia coli) interacts with the C3 hydroxyl in the transition state and ...
|
|
51429
|
His-357 of beta-galactosidase (Escherichia coli) interacts with the C3 hydroxyl in the transition state and ...
|
|
51430
|
His-357 of beta-galactosidase (Escherichia coli) interacts with the C3 hydroxyl in the transition state and ...
|
|
51431
|
His-357 of beta-galactosidase (Escherichia coli) interacts with the C3 hydroxyl in the transition state and ...
|
|
51432
|
His-357 of beta-galactosidase (Escherichia coli) interacts with the C3 hydroxyl in the transition state and ...
|
|
51433
|
His-357 of beta-galactosidase (Escherichia coli) interacts with the C3 hydroxyl in the transition state and ...
|
|
51434
|
His-357 of beta-galactosidase (Escherichia coli) interacts with the C3 hydroxyl in the transition state and ...
|
|
51435
|
His-357 of beta-galactosidase (Escherichia coli) interacts with the C3 hydroxyl in the transition state and ...
|
|
51436
|
His-357 of beta-galactosidase (Escherichia coli) interacts with the C3 hydroxyl in the transition state and ...
|
|
51437
|
His-357 of beta-galactosidase (Escherichia coli) interacts with the C3 hydroxyl in the transition state and ...
|
|
51438
|
His-357 of beta-galactosidase (Escherichia coli) interacts with the C3 hydroxyl in the transition state and ...
|
|
51439
|
His-357 of beta-galactosidase (Escherichia coli) interacts with the C3 hydroxyl in the transition state and ...
|
|
51440
|
His-357 of beta-galactosidase (Escherichia coli) interacts with the C3 hydroxyl in the transition state and ...
|
|
51441
|
His-357 of beta-galactosidase (Escherichia coli) interacts with the C3 hydroxyl in the transition state and ...
|
|
51442
|
His-357 of beta-galactosidase (Escherichia coli) interacts with the C3 hydroxyl in the transition state and ...
|
|
51443
|
His-357 of beta-galactosidase (Escherichia coli) interacts with the C3 hydroxyl in the transition state and ...
|
|
51444
|
His-357 of beta-galactosidase (Escherichia coli) interacts with the C3 hydroxyl in the transition state and ...
|
|
51445
|
His-357 of beta-galactosidase (Escherichia coli) interacts with the C3 hydroxyl in the transition state and ...
|
|
51446
|
His-357 of beta-galactosidase (Escherichia coli) interacts with the C3 hydroxyl in the transition state and ...
|
|
51447
|
His-357 of beta-galactosidase (Escherichia coli) interacts with the C3 hydroxyl in the transition state and ...
|
|
51448
|
His-357 of beta-galactosidase (Escherichia coli) interacts with the C3 hydroxyl in the transition state and ...
|
|
51449
|
His-357 of beta-galactosidase (Escherichia coli) interacts with the C3 hydroxyl in the transition state and ...
|
|
51450
|
His-357 of beta-galactosidase (Escherichia coli) interacts with the C3 hydroxyl in the transition state and ...
|
|
51451
|
His-357 of beta-galactosidase (Escherichia coli) interacts with the C3 hydroxyl in the transition state and ...
|
|
51452
|
His-357 of beta-galactosidase (Escherichia coli) interacts with the C3 hydroxyl in the transition state and ...
|
|
51453
|
His-357 of beta-galactosidase (Escherichia coli) interacts with the C3 hydroxyl in the transition state and ...
|
|
51454
|
His-357 of beta-galactosidase (Escherichia coli) interacts with the C3 hydroxyl in the transition state and ...
|
|
51455
|
His-357 of beta-galactosidase (Escherichia coli) interacts with the C3 hydroxyl in the transition state and ...
|
|
51456
|
His-357 of beta-galactosidase (Escherichia coli) interacts with the C3 hydroxyl in the transition state and ...
|
|
51457
|
His-357 of beta-galactosidase (Escherichia coli) interacts with the C3 hydroxyl in the transition state and ...
|
|
51458
|
His-357 of beta-galactosidase (Escherichia coli) interacts with the C3 hydroxyl in the transition state and ...
|
|
51459
|
His-357 of beta-galactosidase (Escherichia coli) interacts with the C3 hydroxyl in the transition state and ...
|
|
51460
|
His-357 of beta-galactosidase (Escherichia coli) interacts with the C3 hydroxyl in the transition state and ...
|
|
51461
|
His-357 of beta-galactosidase (Escherichia coli) interacts with the C3 hydroxyl in the transition state and ...
|
|
51462
|
His-357 of beta-galactosidase (Escherichia coli) interacts with the C3 hydroxyl in the transition state and ...
|
|
51463
|
His-357 of beta-galactosidase (Escherichia coli) interacts with the C3 hydroxyl in the transition state and ...
|
|
51464
|
His-357 of beta-galactosidase (Escherichia coli) interacts with the C3 hydroxyl in the transition state and ...
|
|
51465
|
His-357 of beta-galactosidase (Escherichia coli) interacts with the C3 hydroxyl in the transition state and ...
|
|
51466
|
His-357 of beta-galactosidase (Escherichia coli) interacts with the C3 hydroxyl in the transition state and ...
|
|
51467
|
His-357 of beta-galactosidase (Escherichia coli) interacts with the C3 hydroxyl in the transition state and ...
|
|
51468
|
His-357 of beta-galactosidase (Escherichia coli) interacts with the C3 hydroxyl in the transition state and ...
|
|
51469
|
His-357 of beta-galactosidase (Escherichia coli) interacts with the C3 hydroxyl in the transition state and ...
|
|
51470
|
His-357 of beta-galactosidase (Escherichia coli) interacts with the C3 hydroxyl in the transition state and ...
|
|
51471
|
His-357 of beta-galactosidase (Escherichia coli) interacts with the C3 hydroxyl in the transition state and ...
|
|
51472
|
His-357 of beta-galactosidase (Escherichia coli) interacts with the C3 hydroxyl in the transition state and ...
|
|
51473
|
His-357 of beta-galactosidase (Escherichia coli) interacts with the C3 hydroxyl in the transition state and ...
|
|
51474
|
His-357 of beta-galactosidase (Escherichia coli) interacts with the C3 hydroxyl in the transition state and ...
|
|
51475
|
His-357 of beta-galactosidase (Escherichia coli) interacts with the C3 hydroxyl in the transition state and ...
|
|
51476
|
His-357 of beta-galactosidase (Escherichia coli) interacts with the C3 hydroxyl in the transition state and ...
|
|
51477
|
His-357 of beta-galactosidase (Escherichia coli) interacts with the C3 hydroxyl in the transition state and ...
|
|
51478
|
His-357 of beta-galactosidase (Escherichia coli) interacts with the C3 hydroxyl in the transition state and ...
|
|
51479
|
His-357 of beta-galactosidase (Escherichia coli) interacts with the C3 hydroxyl in the transition state and ...
|
|
51480
|
His-357 of beta-galactosidase (Escherichia coli) interacts with the C3 hydroxyl in the transition state and ...
|
|
51481
|
His-357 of beta-galactosidase (Escherichia coli) interacts with the C3 hydroxyl in the transition state and ...
|
|
51482
|
His-357 of beta-galactosidase (Escherichia coli) interacts with the C3 hydroxyl in the transition state and ...
|
|
51483
|
His-357 of beta-galactosidase (Escherichia coli) interacts with the C3 hydroxyl in the transition state and ...
|
|
51484
|
His-357 of beta-galactosidase (Escherichia coli) interacts with the C3 hydroxyl in the transition state and ...
|
|
51485
|
His-357 of beta-galactosidase (Escherichia coli) interacts with the C3 hydroxyl in the transition state and ...
|
|
51486
|
His-357 of beta-galactosidase (Escherichia coli) interacts with the C3 hydroxyl in the transition state and ...
|
|
51487
|
His-357 of beta-galactosidase (Escherichia coli) interacts with the C3 hydroxyl in the transition state and ...
|
|
51488
|
His-357 of beta-galactosidase (Escherichia coli) interacts with the C3 hydroxyl in the transition state and ...
|
|
51489
|
His-357 of beta-galactosidase (Escherichia coli) interacts with the C3 hydroxyl in the transition state and ...
|
|
51490
|
His-357 of beta-galactosidase (Escherichia coli) interacts with the C3 hydroxyl in the transition state and ...
|
|
51491
|
His-357 of beta-galactosidase (Escherichia coli) interacts with the C3 hydroxyl in the transition state and ...
|
|
51492
|
His-357 of beta-galactosidase (Escherichia coli) interacts with the C3 hydroxyl in the transition state and ...
|
|
51493
|
His-357 of beta-galactosidase (Escherichia coli) interacts with the C3 hydroxyl in the transition state and ...
|
|
51494
|
His-357 of beta-galactosidase (Escherichia coli) interacts with the C3 hydroxyl in the transition state and ...
|
|
51495
|
His-357 of beta-galactosidase (Escherichia coli) interacts with the C3 hydroxyl in the transition state and ...
|
|
51496
|
His-357 of beta-galactosidase (Escherichia coli) interacts with the C3 hydroxyl in the transition state and ...
|
|
51497
|
His-357 of beta-galactosidase (Escherichia coli) interacts with the C3 hydroxyl in the transition state and ...
|
|
51498
|
His-357 of beta-galactosidase (Escherichia coli) interacts with the C3 hydroxyl in the transition state and ...
|
|
51499
|
His-357 of beta-galactosidase (Escherichia coli) interacts with the C3 hydroxyl in the transition state and ...
|
|
51500
|
His-357 of beta-galactosidase (Escherichia coli) interacts with the C3 hydroxyl in the transition state and ...
|
|
51501
|
His-357 of beta-galactosidase (Escherichia coli) interacts with the C3 hydroxyl in the transition state and ...
|
|
51502
|
His-357 of beta-galactosidase (Escherichia coli) interacts with the C3 hydroxyl in the transition state and ...
|
|
51503
|
His-357 of beta-galactosidase (Escherichia coli) interacts with the C3 hydroxyl in the transition state and ...
|
|
51504
|
His-357 of beta-galactosidase (Escherichia coli) interacts with the C3 hydroxyl in the transition state and ...
|
|
51505
|
His-357 of beta-galactosidase (Escherichia coli) interacts with the C3 hydroxyl in the transition state and ...
|
|
51506
|
His-357 of beta-galactosidase (Escherichia coli) interacts with the C3 hydroxyl in the transition state and ...
|
|
51507
|
His-357 of beta-galactosidase (Escherichia coli) interacts with the C3 hydroxyl in the transition state and ...
|
|
51508
|
His-357 of beta-galactosidase (Escherichia coli) interacts with the C3 hydroxyl in the transition state and ...
|
|
51509
|
His-357 of beta-galactosidase (Escherichia coli) interacts with the C3 hydroxyl in the transition state and ...
|
|
51510
|
His-357 of beta-galactosidase (Escherichia coli) interacts with the C3 hydroxyl in the transition state and ...
|
|
51511
|
His-357 of beta-galactosidase (Escherichia coli) interacts with the C3 hydroxyl in the transition state and ...
|
|
51512
|
His-357 of beta-galactosidase (Escherichia coli) interacts with the C3 hydroxyl in the transition state and ...
|
|
51513
|
His-357 of beta-galactosidase (Escherichia coli) interacts with the C3 hydroxyl in the transition state and ...
|
|
51514
|
His-357 of beta-galactosidase (Escherichia coli) interacts with the C3 hydroxyl in the transition state and ...
|
|
51515
|
His-357 of beta-galactosidase (Escherichia coli) interacts with the C3 hydroxyl in the transition state and ...
|
|
51516
|
His-357 of beta-galactosidase (Escherichia coli) interacts with the C3 hydroxyl in the transition state and ...
|
|
51517
|
His-357 of beta-galactosidase (Escherichia coli) interacts with the C3 hydroxyl in the transition state and ...
|
|
51518
|
His-357 of beta-galactosidase (Escherichia coli) interacts with the C3 hydroxyl in the transition state and ...
|
|
51519
|
His-357 of beta-galactosidase (Escherichia coli) interacts with the C3 hydroxyl in the transition state and ...
|
|
51520
|
His-357 of beta-galactosidase (Escherichia coli) interacts with the C3 hydroxyl in the transition state and ...
|
|
51521
|
His-357 of beta-galactosidase (Escherichia coli) interacts with the C3 hydroxyl in the transition state and ...
|
|
51522
|
His-357 of beta-galactosidase (Escherichia coli) interacts with the C3 hydroxyl in the transition state and ...
|
|
51523
|
His-357 of beta-galactosidase (Escherichia coli) interacts with the C3 hydroxyl in the transition state and ...
|
|
51524
|
His-357 of beta-galactosidase (Escherichia coli) interacts with the C3 hydroxyl in the transition state and ...
|
|
51525
|
His-357 of beta-galactosidase (Escherichia coli) interacts with the C3 hydroxyl in the transition state and ...
|
|
51526
|
His-357 of beta-galactosidase (Escherichia coli) interacts with the C3 hydroxyl in the transition state and ...
|
|
51527
|
His-357 of beta-galactosidase (Escherichia coli) interacts with the C3 hydroxyl in the transition state and ...
|
|
51528
|
His-357 of beta-galactosidase (Escherichia coli) interacts with the C3 hydroxyl in the transition state and ...
|
|
51529
|
His-357 of beta-galactosidase (Escherichia coli) interacts with the C3 hydroxyl in the transition state and ...
|
|
51530
|
His-357 of beta-galactosidase (Escherichia coli) interacts with the C3 hydroxyl in the transition state and ...
|
|
51531
|
His-357 of beta-galactosidase (Escherichia coli) interacts with the C3 hydroxyl in the transition state and ...
|
|
51532
|
His-357 of beta-galactosidase (Escherichia coli) interacts with the C3 hydroxyl in the transition state and ...
|
|
51533
|
His-357 of beta-galactosidase (Escherichia coli) interacts with the C3 hydroxyl in the transition state and ...
|
|
51534
|
His-357 of beta-galactosidase (Escherichia coli) interacts with the C3 hydroxyl in the transition state and ...
|
|
51535
|
His-357 of beta-galactosidase (Escherichia coli) interacts with the C3 hydroxyl in the transition state and ...
|
|
51536
|
His-357 of beta-galactosidase (Escherichia coli) interacts with the C3 hydroxyl in the transition state and ...
|
|
51537
|
His-357 of beta-galactosidase (Escherichia coli) interacts with the C3 hydroxyl in the transition state and ...
|
|
51538
|
His-357 of beta-galactosidase (Escherichia coli) interacts with the C3 hydroxyl in the transition state and ...
|
|
51539
|
His-357 of beta-galactosidase (Escherichia coli) interacts with the C3 hydroxyl in the transition state and ...
|
|
51540
|
His-357 of beta-galactosidase (Escherichia coli) interacts with the C3 hydroxyl in the transition state and ...
|
|
51541
|
His-357 of beta-galactosidase (Escherichia coli) interacts with the C3 hydroxyl in the transition state and ...
|
|
51542
|
His-357 of beta-galactosidase (Escherichia coli) interacts with the C3 hydroxyl in the transition state and ...
|
|
51543
|
His-357 of beta-galactosidase (Escherichia coli) interacts with the C3 hydroxyl in the transition state and ...
|
|
51544
|
His-357 of beta-galactosidase (Escherichia coli) interacts with the C3 hydroxyl in the transition state and ...
|
|
51545
|
His-357 of beta-galactosidase (Escherichia coli) interacts with the C3 hydroxyl in the transition state and ...
|
|
51546
|
His-357 of beta-galactosidase (Escherichia coli) interacts with the C3 hydroxyl in the transition state and ...
|
|
51547
|
His-357 of beta-galactosidase (Escherichia coli) interacts with the C3 hydroxyl in the transition state and ...
|
|
51548
|
His-357 of beta-galactosidase (Escherichia coli) interacts with the C3 hydroxyl in the transition state and ...
|
|
51549
|
His-357 of beta-galactosidase (Escherichia coli) interacts with the C3 hydroxyl in the transition state and ...
|
|
51550
|
His-357 of beta-galactosidase (Escherichia coli) interacts with the C3 hydroxyl in the transition state and ...
|
|
51551
|
His-357 of beta-galactosidase (Escherichia coli) interacts with the C3 hydroxyl in the transition state and ...
|
|
51552
|
His-357 of beta-galactosidase (Escherichia coli) interacts with the C3 hydroxyl in the transition state and ...
|
|
51553
|
His-357 of beta-galactosidase (Escherichia coli) interacts with the C3 hydroxyl in the transition state and ...
|
|
51554
|
His-357 of beta-galactosidase (Escherichia coli) interacts with the C3 hydroxyl in the transition state and ...
|
|
51555
|
His-357 of beta-galactosidase (Escherichia coli) interacts with the C3 hydroxyl in the transition state and ...
|
|
51556
|
His-357 of beta-galactosidase (Escherichia coli) interacts with the C3 hydroxyl in the transition state and ...
|
|
51557
|
His-357 of beta-galactosidase (Escherichia coli) interacts with the C3 hydroxyl in the transition state and ...
|
|
51558
|
His-357 of beta-galactosidase (Escherichia coli) interacts with the C3 hydroxyl in the transition state and ...
|
|
51559
|
Inhibition of influenza A virus sialidase activity by sulfatide
|
|
51560
|
Inhibition of influenza A virus sialidase activity by sulfatide
|
|
51561
|
Inhibition of influenza A virus sialidase activity by sulfatide
|
|
51562
|
Inhibition of influenza A virus sialidase activity by sulfatide
|
|
51563
|
Inhibition of influenza A virus sialidase activity by sulfatide
|
|
51564
|
Inhibition of influenza A virus sialidase activity by sulfatide
|
|
51565
|
Regulation of NAD- and NADP-dependent isocitrate dehydrogenases by reduction levels of pyridine nucleotides in ...
|
|
51566
|
Regulation of NAD- and NADP-dependent isocitrate dehydrogenases by reduction levels of pyridine nucleotides in ...
|
|
51567
|
Regulation of NAD- and NADP-dependent isocitrate dehydrogenases by reduction levels of pyridine nucleotides in ...
|
|
51568
|
Regulation of NAD- and NADP-dependent isocitrate dehydrogenases by reduction levels of pyridine nucleotides in ...
|
|
51569
|
Regulation of NAD- and NADP-dependent isocitrate dehydrogenases by reduction levels of pyridine nucleotides in ...
|
|
51570
|
Regulation of NAD- and NADP-dependent isocitrate dehydrogenases by reduction levels of pyridine nucleotides in ...
|
|
51571
|
Regulation of NAD- and NADP-dependent isocitrate dehydrogenases by reduction levels of pyridine nucleotides in ...
|
|
51572
|
Regulation of NAD- and NADP-dependent isocitrate dehydrogenases by reduction levels of pyridine nucleotides in ...
|
|
51573
|
Divergent roles in Arabidopsis thaliana development and defense of two homologous genes, aberrant growth and ...
|
|
51574
|
Divergent roles in Arabidopsis thaliana development and defense of two homologous genes, aberrant growth and ...
|
|
51575
|
Divergent roles in Arabidopsis thaliana development and defense of two homologous genes, aberrant growth and ...
|
|
51576
|
Divergent roles in Arabidopsis thaliana development and defense of two homologous genes, aberrant growth and ...
|
|
51577
|
Divergent roles in Arabidopsis thaliana development and defense of two homologous genes, aberrant growth and ...
|
|
51578
|
Divergent roles in Arabidopsis thaliana development and defense of two homologous genes, aberrant growth and ...
|
|
51579
|
Divergent roles in Arabidopsis thaliana development and defense of two homologous genes, aberrant growth and ...
|
|
51580
|
Divergent roles in Arabidopsis thaliana development and defense of two homologous genes, aberrant growth and ...
|
|
51581
|
Divergent roles in Arabidopsis thaliana development and defense of two homologous genes, aberrant growth and ...
|
|
51582
|
Divergent roles in Arabidopsis thaliana development and defense of two homologous genes, aberrant growth and ...
|
|
51583
|
Divergent roles in Arabidopsis thaliana development and defense of two homologous genes, aberrant growth and ...
|
|
51584
|
Divergent roles in Arabidopsis thaliana development and defense of two homologous genes, aberrant growth and ...
|
|
51585
|
Divergent roles in Arabidopsis thaliana development and defense of two homologous genes, aberrant growth and ...
|
|
51586
|
Divergent roles in Arabidopsis thaliana development and defense of two homologous genes, aberrant growth and ...
|
|
51587
|
Divergent roles in Arabidopsis thaliana development and defense of two homologous genes, aberrant growth and ...
|
|
51588
|
A feedback-resistant mutant of Bacillus subtilis glutamine synthetase with pleiotropic defects in ...
|
|
51589
|
A feedback-resistant mutant of Bacillus subtilis glutamine synthetase with pleiotropic defects in ...
|
|
51590
|
A feedback-resistant mutant of Bacillus subtilis glutamine synthetase with pleiotropic defects in ...
|
|
51591
|
A feedback-resistant mutant of Bacillus subtilis glutamine synthetase with pleiotropic defects in ...
|
|
51592
|
A feedback-resistant mutant of Bacillus subtilis glutamine synthetase with pleiotropic defects in ...
|
|
51593
|
A feedback-resistant mutant of Bacillus subtilis glutamine synthetase with pleiotropic defects in ...
|
|
51594
|
A feedback-resistant mutant of Bacillus subtilis glutamine synthetase with pleiotropic defects in ...
|
|
51595
|
A feedback-resistant mutant of Bacillus subtilis glutamine synthetase with pleiotropic defects in ...
|
|
51596
|
A feedback-resistant mutant of Bacillus subtilis glutamine synthetase with pleiotropic defects in ...
|
|
51597
|
A feedback-resistant mutant of Bacillus subtilis glutamine synthetase with pleiotropic defects in ...
|
|
51598
|
A feedback-resistant mutant of Bacillus subtilis glutamine synthetase with pleiotropic defects in ...
|
|
51599
|
A feedback-resistant mutant of Bacillus subtilis glutamine synthetase with pleiotropic defects in ...
|
|
51600
|
A feedback-resistant mutant of Bacillus subtilis glutamine synthetase with pleiotropic defects in ...
|
|
51601
|
A feedback-resistant mutant of Bacillus subtilis glutamine synthetase with pleiotropic defects in ...
|
|
51602
|
A feedback-resistant mutant of Bacillus subtilis glutamine synthetase with pleiotropic defects in ...
|
|
51603
|
A feedback-resistant mutant of Bacillus subtilis glutamine synthetase with pleiotropic defects in ...
|
|
51604
|
A feedback-resistant mutant of Bacillus subtilis glutamine synthetase with pleiotropic defects in ...
|
|
51605
|
A feedback-resistant mutant of Bacillus subtilis glutamine synthetase with pleiotropic defects in ...
|
|
51606
|
A feedback-resistant mutant of Bacillus subtilis glutamine synthetase with pleiotropic defects in ...
|
|
51607
|
A feedback-resistant mutant of Bacillus subtilis glutamine synthetase with pleiotropic defects in ...
|
|
51608
|
A feedback-resistant mutant of Bacillus subtilis glutamine synthetase with pleiotropic defects in ...
|
|
51609
|
A feedback-resistant mutant of Bacillus subtilis glutamine synthetase with pleiotropic defects in ...
|
|
51610
|
A feedback-resistant mutant of Bacillus subtilis glutamine synthetase with pleiotropic defects in ...
|
|
51611
|
Development of a retinoic acid receptor-binding assay with rainbow trout tissue: characterization of retinoic ...
|
|
51612
|
Development of a retinoic acid receptor-binding assay with rainbow trout tissue: characterization of retinoic ...
|
|
51613
|
Development of a retinoic acid receptor-binding assay with rainbow trout tissue: characterization of retinoic ...
|
|
51614
|
Development of a retinoic acid receptor-binding assay with rainbow trout tissue: characterization of retinoic ...
|
|
51615
|
Development of a retinoic acid receptor-binding assay with rainbow trout tissue: characterization of retinoic ...
|
|
51616
|
Development of a retinoic acid receptor-binding assay with rainbow trout tissue: characterization of retinoic ...
|
|
51617
|
Development of a retinoic acid receptor-binding assay with rainbow trout tissue: characterization of retinoic ...
|
|
51618
|
Development of a retinoic acid receptor-binding assay with rainbow trout tissue: characterization of retinoic ...
|
|
51619
|
Development of a retinoic acid receptor-binding assay with rainbow trout tissue: characterization of retinoic ...
|
|
51620
|
Development of a retinoic acid receptor-binding assay with rainbow trout tissue: characterization of retinoic ...
|
|
51621
|
Development of a retinoic acid receptor-binding assay with rainbow trout tissue: characterization of retinoic ...
|
|
51622
|
Development of a retinoic acid receptor-binding assay with rainbow trout tissue: characterization of retinoic ...
|
|
51623
|
Development of a retinoic acid receptor-binding assay with rainbow trout tissue: characterization of retinoic ...
|
|
51624
|
Development of a retinoic acid receptor-binding assay with rainbow trout tissue: characterization of retinoic ...
|
|
51625
|
Development of a retinoic acid receptor-binding assay with rainbow trout tissue: characterization of retinoic ...
|
|
51626
|
Development of a retinoic acid receptor-binding assay with rainbow trout tissue: characterization of retinoic ...
|
|
51627
|
Development of a retinoic acid receptor-binding assay with rainbow trout tissue: characterization of retinoic ...
|
|
51628
|
Development of a retinoic acid receptor-binding assay with rainbow trout tissue: characterization of retinoic ...
|
|
51629
|
Development of a retinoic acid receptor-binding assay with rainbow trout tissue: characterization of retinoic ...
|
|
51630
|
Development of a retinoic acid receptor-binding assay with rainbow trout tissue: characterization of retinoic ...
|
|
51631
|
L-myo-lnositol 1-Phosphate Synthase from Plant Sources (Characteristics of the Chloroplastic and Cytosolic ...
|
|
51632
|
L-myo-lnositol 1-Phosphate Synthase from Plant Sources (Characteristics of the Chloroplastic and Cytosolic ...
|
|
51633
|
L-myo-lnositol 1-Phosphate Synthase from Plant Sources (Characteristics of the Chloroplastic and Cytosolic ...
|
|
51634
|
L-myo-lnositol 1-Phosphate Synthase from Plant Sources (Characteristics of the Chloroplastic and Cytosolic ...
|
|
51635
|
L-myo-lnositol 1-Phosphate Synthase from Plant Sources (Characteristics of the Chloroplastic and Cytosolic ...
|
|
51636
|
L-myo-lnositol 1-Phosphate Synthase from Plant Sources (Characteristics of the Chloroplastic and Cytosolic ...
|
|
51637
|
L-myo-lnositol 1-Phosphate Synthase from Plant Sources (Characteristics of the Chloroplastic and Cytosolic ...
|
|
51638
|
L-myo-lnositol 1-Phosphate Synthase from Plant Sources (Characteristics of the Chloroplastic and Cytosolic ...
|
|
51639
|
L-myo-lnositol 1-Phosphate Synthase from Plant Sources (Characteristics of the Chloroplastic and Cytosolic ...
|
|
51640
|
L-myo-lnositol 1-Phosphate Synthase from Plant Sources (Characteristics of the Chloroplastic and Cytosolic ...
|
|
51641
|
L-myo-lnositol 1-Phosphate Synthase from Plant Sources (Characteristics of the Chloroplastic and Cytosolic ...
|
|
51642
|
L-myo-lnositol 1-Phosphate Synthase from Plant Sources (Characteristics of the Chloroplastic and Cytosolic ...
|
|
51643
|
L-myo-lnositol 1-Phosphate Synthase from Plant Sources (Characteristics of the Chloroplastic and Cytosolic ...
|
|
51644
|
L-myo-lnositol 1-Phosphate Synthase from Plant Sources (Characteristics of the Chloroplastic and Cytosolic ...
|
|
51645
|
L-myo-lnositol 1-Phosphate Synthase from Plant Sources (Characteristics of the Chloroplastic and Cytosolic ...
|
|
51646
|
L-myo-lnositol 1-Phosphate Synthase from Plant Sources (Characteristics of the Chloroplastic and Cytosolic ...
|
|
51647
|
The characterization of mutant Bacillus subtilis adenylosuccinate lyases corresponding to severe human ...
|
|
51648
|
The characterization of mutant Bacillus subtilis adenylosuccinate lyases corresponding to severe human ...
|
|
51649
|
The characterization of mutant Bacillus subtilis adenylosuccinate lyases corresponding to severe human ...
|
|
51650
|
The characterization of mutant Bacillus subtilis adenylosuccinate lyases corresponding to severe human ...
|
|
51651
|
The characterization of mutant Bacillus subtilis adenylosuccinate lyases corresponding to severe human ...
|
|
51652
|
The characterization of mutant Bacillus subtilis adenylosuccinate lyases corresponding to severe human ...
|
|
51653
|
The characterization of mutant Bacillus subtilis adenylosuccinate lyases corresponding to severe human ...
|
|
51654
|
The characterization of mutant Bacillus subtilis adenylosuccinate lyases corresponding to severe human ...
|
|
51655
|
The characterization of mutant Bacillus subtilis adenylosuccinate lyases corresponding to severe human ...
|
|
51656
|
The characterization of mutant Bacillus subtilis adenylosuccinate lyases corresponding to severe human ...
|
|
51657
|
The characterization of mutant Bacillus subtilis adenylosuccinate lyases corresponding to severe human ...
|
|
51658
|
The characterization of mutant Bacillus subtilis adenylosuccinate lyases corresponding to severe human ...
|
|
51659
|
The characterization of mutant Bacillus subtilis adenylosuccinate lyases corresponding to severe human ...
|
|
51660
|
The characterization of mutant Bacillus subtilis adenylosuccinate lyases corresponding to severe human ...
|
|
51661
|
The characterization of mutant Bacillus subtilis adenylosuccinate lyases corresponding to severe human ...
|
|
51662
|
The characterization of mutant Bacillus subtilis adenylosuccinate lyases corresponding to severe human ...
|
|
51663
|
The characterization of mutant Bacillus subtilis adenylosuccinate lyases corresponding to severe human ...
|
|
51664
|
The characterization of mutant Bacillus subtilis adenylosuccinate lyases corresponding to severe human ...
|
|
51665
|
A colorimetric determination of inositol monophosphates as an assay for D-glucose 6-phosphate-1L-myoinositol ...
|
|
51666
|
A colorimetric determination of inositol monophosphates as an assay for D-glucose 6-phosphate-1L-myoinositol ...
|
|
51667
|
Abscisic aldehyde oxidase in leaves of Arabidopsis thaliana
|
|
51668
|
Abscisic aldehyde oxidase in leaves of Arabidopsis thaliana
|
|
51669
|
Abscisic aldehyde oxidase in leaves of Arabidopsis thaliana
|
|
51670
|
Abscisic aldehyde oxidase in leaves of Arabidopsis thaliana
|
|
51671
|
Abscisic aldehyde oxidase in leaves of Arabidopsis thaliana
|
|
51672
|
Proposed mechanism and functional amino acid residues of malonyl-CoA:anthocyanin ...
|
|
51673
|
Proposed mechanism and functional amino acid residues of malonyl-CoA:anthocyanin ...
|
|
51674
|
Proposed mechanism and functional amino acid residues of malonyl-CoA:anthocyanin ...
|
|
51675
|
Proposed mechanism and functional amino acid residues of malonyl-CoA:anthocyanin ...
|
|
51676
|
Proposed mechanism and functional amino acid residues of malonyl-CoA:anthocyanin ...
|
|
51677
|
Proposed mechanism and functional amino acid residues of malonyl-CoA:anthocyanin ...
|
|
51678
|
Proposed mechanism and functional amino acid residues of malonyl-CoA:anthocyanin ...
|
|
51679
|
Proposed mechanism and functional amino acid residues of malonyl-CoA:anthocyanin ...
|
|
51680
|
Proposed mechanism and functional amino acid residues of malonyl-CoA:anthocyanin ...
|
|
51681
|
Maize phosphoenolpyruvate carboxylase. Mutations at the putative binding site for glucose 6-phosphate caused ...
|
|
51682
|
Maize phosphoenolpyruvate carboxylase. Mutations at the putative binding site for glucose 6-phosphate caused ...
|
|
51683
|
Maize phosphoenolpyruvate carboxylase. Mutations at the putative binding site for glucose 6-phosphate caused ...
|
|
51684
|
Maize phosphoenolpyruvate carboxylase. Mutations at the putative binding site for glucose 6-phosphate caused ...
|
|
51685
|
Maize phosphoenolpyruvate carboxylase. Mutations at the putative binding site for glucose 6-phosphate caused ...
|
|
51686
|
Maize phosphoenolpyruvate carboxylase. Mutations at the putative binding site for glucose 6-phosphate caused ...
|
|
51687
|
Maize phosphoenolpyruvate carboxylase. Mutations at the putative binding site for glucose 6-phosphate caused ...
|
|
51688
|
Maize phosphoenolpyruvate carboxylase. Mutations at the putative binding site for glucose 6-phosphate caused ...
|
|
51689
|
Maize phosphoenolpyruvate carboxylase. Mutations at the putative binding site for glucose 6-phosphate caused ...
|
|
51690
|
Maize phosphoenolpyruvate carboxylase. Mutations at the putative binding site for glucose 6-phosphate caused ...
|
|
51691
|
Maize phosphoenolpyruvate carboxylase. Mutations at the putative binding site for glucose 6-phosphate caused ...
|
|
51692
|
Maize phosphoenolpyruvate carboxylase. Mutations at the putative binding site for glucose 6-phosphate caused ...
|
|
51693
|
Maize phosphoenolpyruvate carboxylase. Mutations at the putative binding site for glucose 6-phosphate caused ...
|
|
51694
|
Maize phosphoenolpyruvate carboxylase. Mutations at the putative binding site for glucose 6-phosphate caused ...
|
|
51695
|
Maize phosphoenolpyruvate carboxylase. Mutations at the putative binding site for glucose 6-phosphate caused ...
|
|
51696
|
Maize phosphoenolpyruvate carboxylase. Mutations at the putative binding site for glucose 6-phosphate caused ...
|
|
51697
|
Maize phosphoenolpyruvate carboxylase. Mutations at the putative binding site for glucose 6-phosphate caused ...
|
|
51698
|
Maize phosphoenolpyruvate carboxylase. Mutations at the putative binding site for glucose 6-phosphate caused ...
|
|
51699
|
Maize phosphoenolpyruvate carboxylase. Mutations at the putative binding site for glucose 6-phosphate caused ...
|
|
51700
|
Maize phosphoenolpyruvate carboxylase. Mutations at the putative binding site for glucose 6-phosphate caused ...
|
|
51701
|
Maize phosphoenolpyruvate carboxylase. Mutations at the putative binding site for glucose 6-phosphate caused ...
|
|
51702
|
Maize phosphoenolpyruvate carboxylase. Mutations at the putative binding site for glucose 6-phosphate caused ...
|
|
51703
|
Maize phosphoenolpyruvate carboxylase. Mutations at the putative binding site for glucose 6-phosphate caused ...
|
|
51704
|
Maize phosphoenolpyruvate carboxylase. Mutations at the putative binding site for glucose 6-phosphate caused ...
|
|
51705
|
Maize phosphoenolpyruvate carboxylase. Mutations at the putative binding site for glucose 6-phosphate caused ...
|
|
51706
|
Maize phosphoenolpyruvate carboxylase. Mutations at the putative binding site for glucose 6-phosphate caused ...
|
|
51707
|
Maize phosphoenolpyruvate carboxylase. Mutations at the putative binding site for glucose 6-phosphate caused ...
|
|
51708
|
Maize phosphoenolpyruvate carboxylase. Mutations at the putative binding site for glucose 6-phosphate caused ...
|
|
51709
|
Maize phosphoenolpyruvate carboxylase. Mutations at the putative binding site for glucose 6-phosphate caused ...
|
|
51710
|
Maize phosphoenolpyruvate carboxylase. Mutations at the putative binding site for glucose 6-phosphate caused ...
|
|
51711
|
Maize phosphoenolpyruvate carboxylase. Mutations at the putative binding site for glucose 6-phosphate caused ...
|
|
51712
|
Maize phosphoenolpyruvate carboxylase. Mutations at the putative binding site for glucose 6-phosphate caused ...
|
|
51713
|
Maize phosphoenolpyruvate carboxylase. Mutations at the putative binding site for glucose 6-phosphate caused ...
|
|
51714
|
Maize phosphoenolpyruvate carboxylase. Mutations at the putative binding site for glucose 6-phosphate caused ...
|
|
51715
|
Maize phosphoenolpyruvate carboxylase. Mutations at the putative binding site for glucose 6-phosphate caused ...
|
|
51716
|
Maize phosphoenolpyruvate carboxylase. Mutations at the putative binding site for glucose 6-phosphate caused ...
|
|
51717
|
Maize phosphoenolpyruvate carboxylase. Mutations at the putative binding site for glucose 6-phosphate caused ...
|
|
51718
|
Maize phosphoenolpyruvate carboxylase. Mutations at the putative binding site for glucose 6-phosphate caused ...
|
|
51719
|
Maize phosphoenolpyruvate carboxylase. Mutations at the putative binding site for glucose 6-phosphate caused ...
|
|
51720
|
Maize phosphoenolpyruvate carboxylase. Mutations at the putative binding site for glucose 6-phosphate caused ...
|
|
51721
|
Maize phosphoenolpyruvate carboxylase. Mutations at the putative binding site for glucose 6-phosphate caused ...
|
|
51722
|
Maize phosphoenolpyruvate carboxylase. Mutations at the putative binding site for glucose 6-phosphate caused ...
|
|
51723
|
Maize phosphoenolpyruvate carboxylase. Mutations at the putative binding site for glucose 6-phosphate caused ...
|
|
51724
|
Maize phosphoenolpyruvate carboxylase. Mutations at the putative binding site for glucose 6-phosphate caused ...
|
|
51725
|
Maize phosphoenolpyruvate carboxylase. Mutations at the putative binding site for glucose 6-phosphate caused ...
|
|
51726
|
Maize phosphoenolpyruvate carboxylase. Mutations at the putative binding site for glucose 6-phosphate caused ...
|
|
51727
|
Maize phosphoenolpyruvate carboxylase. Mutations at the putative binding site for glucose 6-phosphate caused ...
|
|
51728
|
Maize phosphoenolpyruvate carboxylase. Mutations at the putative binding site for glucose 6-phosphate caused ...
|
|
51729
|
Maize phosphoenolpyruvate carboxylase. Mutations at the putative binding site for glucose 6-phosphate caused ...
|
|
51730
|
Maize phosphoenolpyruvate carboxylase. Mutations at the putative binding site for glucose 6-phosphate caused ...
|
|
51731
|
Maize phosphoenolpyruvate carboxylase. Mutations at the putative binding site for glucose 6-phosphate caused ...
|
|
51732
|
Maize phosphoenolpyruvate carboxylase. Mutations at the putative binding site for glucose 6-phosphate caused ...
|
|
51733
|
Maize phosphoenolpyruvate carboxylase. Mutations at the putative binding site for glucose 6-phosphate caused ...
|
|
51734
|
Maize phosphoenolpyruvate carboxylase. Mutations at the putative binding site for glucose 6-phosphate caused ...
|
|
51735
|
Maize phosphoenolpyruvate carboxylase. Mutations at the putative binding site for glucose 6-phosphate caused ...
|
|
51736
|
Maize phosphoenolpyruvate carboxylase. Mutations at the putative binding site for glucose 6-phosphate caused ...
|
|
51737
|
Maize phosphoenolpyruvate carboxylase. Mutations at the putative binding site for glucose 6-phosphate caused ...
|
|
51738
|
Maize phosphoenolpyruvate carboxylase. Mutations at the putative binding site for glucose 6-phosphate caused ...
|
|
51739
|
Maize phosphoenolpyruvate carboxylase. Mutations at the putative binding site for glucose 6-phosphate caused ...
|
|
51740
|
Maize phosphoenolpyruvate carboxylase. Mutations at the putative binding site for glucose 6-phosphate caused ...
|
|
51741
|
Maize phosphoenolpyruvate carboxylase. Mutations at the putative binding site for glucose 6-phosphate caused ...
|
|
51742
|
Maize phosphoenolpyruvate carboxylase. Mutations at the putative binding site for glucose 6-phosphate caused ...
|
|
51743
|
Maize phosphoenolpyruvate carboxylase. Mutations at the putative binding site for glucose 6-phosphate caused ...
|
|
51744
|
Maize phosphoenolpyruvate carboxylase. Mutations at the putative binding site for glucose 6-phosphate caused ...
|
|
51745
|
Maize phosphoenolpyruvate carboxylase. Mutations at the putative binding site for glucose 6-phosphate caused ...
|
|
51746
|
Maize phosphoenolpyruvate carboxylase. Mutations at the putative binding site for glucose 6-phosphate caused ...
|
|
51747
|
Maize phosphoenolpyruvate carboxylase. Mutations at the putative binding site for glucose 6-phosphate caused ...
|
|
51748
|
Maize phosphoenolpyruvate carboxylase. Mutations at the putative binding site for glucose 6-phosphate caused ...
|
|
51749
|
Maize phosphoenolpyruvate carboxylase. Mutations at the putative binding site for glucose 6-phosphate caused ...
|
|
51750
|
Maize phosphoenolpyruvate carboxylase. Mutations at the putative binding site for glucose 6-phosphate caused ...
|
|
51751
|
Maize phosphoenolpyruvate carboxylase. Mutations at the putative binding site for glucose 6-phosphate caused ...
|
|
51752
|
Maize phosphoenolpyruvate carboxylase. Mutations at the putative binding site for glucose 6-phosphate caused ...
|
|
51753
|
Maize phosphoenolpyruvate carboxylase. Mutations at the putative binding site for glucose 6-phosphate caused ...
|
|
51754
|
Maize phosphoenolpyruvate carboxylase. Mutations at the putative binding site for glucose 6-phosphate caused ...
|
|
51755
|
Maize phosphoenolpyruvate carboxylase. Mutations at the putative binding site for glucose 6-phosphate caused ...
|
|
51756
|
Maize phosphoenolpyruvate carboxylase. Mutations at the putative binding site for glucose 6-phosphate caused ...
|
|
51757
|
Maize phosphoenolpyruvate carboxylase. Mutations at the putative binding site for glucose 6-phosphate caused ...
|
|
51758
|
Maize phosphoenolpyruvate carboxylase. Mutations at the putative binding site for glucose 6-phosphate caused ...
|
|
51759
|
Probing the role of the C-terminus of Bacillus subtilis chorismate mutase by a novel random ...
|
|
51760
|
Probing the role of the C-terminus of Bacillus subtilis chorismate mutase by a novel random ...
|
|
51761
|
Probing the role of the C-terminus of Bacillus subtilis chorismate mutase by a novel random ...
|
|
51762
|
Probing the role of the C-terminus of Bacillus subtilis chorismate mutase by a novel random ...
|
|
51763
|
Probing the role of the C-terminus of Bacillus subtilis chorismate mutase by a novel random ...
|
|
51764
|
Probing the role of the C-terminus of Bacillus subtilis chorismate mutase by a novel random ...
|
|
51765
|
Probing the role of the C-terminus of Bacillus subtilis chorismate mutase by a novel random ...
|
|
51766
|
The Arabidopsis phenylalanine ammonia lyase gene family: kinetic characterization of the four PAL isoforms.
|
|
51767
|
The Arabidopsis phenylalanine ammonia lyase gene family: kinetic characterization of the four PAL isoforms.
|
|
51768
|
The Arabidopsis phenylalanine ammonia lyase gene family: kinetic characterization of the four PAL isoforms.
|
|
51769
|
The Arabidopsis phenylalanine ammonia lyase gene family: kinetic characterization of the four PAL isoforms.
|
|
51770
|
The Arabidopsis phenylalanine ammonia lyase gene family: kinetic characterization of the four PAL isoforms.
|
|
51771
|
The Arabidopsis phenylalanine ammonia lyase gene family: kinetic characterization of the four PAL isoforms.
|
|
51772
|
The Arabidopsis phenylalanine ammonia lyase gene family: kinetic characterization of the four PAL isoforms.
|
|
51773
|
Specificity and enzyme kinetics of the quorum-quenching N-Acyl homoserine lactone lactonase (AHL-lactonase)
|
|
51774
|
Specificity and enzyme kinetics of the quorum-quenching N-Acyl homoserine lactone lactonase (AHL-lactonase)
|
|
51775
|
Specificity and enzyme kinetics of the quorum-quenching N-Acyl homoserine lactone lactonase (AHL-lactonase)
|
|
51776
|
Specificity and enzyme kinetics of the quorum-quenching N-Acyl homoserine lactone lactonase (AHL-lactonase)
|
|
51777
|
Specificity and enzyme kinetics of the quorum-quenching N-Acyl homoserine lactone lactonase (AHL-lactonase)
|
|
51778
|
Specificity and enzyme kinetics of the quorum-quenching N-Acyl homoserine lactone lactonase (AHL-lactonase)
|
|
51779
|
Specificity and enzyme kinetics of the quorum-quenching N-Acyl homoserine lactone lactonase (AHL-lactonase)
|
|
51780
|
Specificity and enzyme kinetics of the quorum-quenching N-Acyl homoserine lactone lactonase (AHL-lactonase)
|
|
51781
|
Specificity and enzyme kinetics of the quorum-quenching N-Acyl homoserine lactone lactonase (AHL-lactonase)
|
|
51782
|
Specificity and enzyme kinetics of the quorum-quenching N-Acyl homoserine lactone lactonase (AHL-lactonase)
|
|
51783
|
Mutational, structural, and kinetic evidence for a dissociative mechanism in the GDP-mannose mannosyl ...
|
|
51784
|
Mutational, structural, and kinetic evidence for a dissociative mechanism in the GDP-mannose mannosyl ...
|
|
51785
|
Mutational, structural, and kinetic evidence for a dissociative mechanism in the GDP-mannose mannosyl ...
|
|
51786
|
Mutational, structural, and kinetic evidence for a dissociative mechanism in the GDP-mannose mannosyl ...
|
|
51787
|
Mutational, structural, and kinetic evidence for a dissociative mechanism in the GDP-mannose mannosyl ...
|
|
51788
|
Mutational, structural, and kinetic evidence for a dissociative mechanism in the GDP-mannose mannosyl ...
|
|
51789
|
Mutational, structural, and kinetic evidence for a dissociative mechanism in the GDP-mannose mannosyl ...
|
|
51790
|
Mutational, structural, and kinetic evidence for a dissociative mechanism in the GDP-mannose mannosyl ...
|
|
51791
|
Mutational, structural, and kinetic evidence for a dissociative mechanism in the GDP-mannose mannosyl ...
|
|
51792
|
Mutational, structural, and kinetic evidence for a dissociative mechanism in the GDP-mannose mannosyl ...
|
|
51793
|
Mutational, structural, and kinetic evidence for a dissociative mechanism in the GDP-mannose mannosyl ...
|
|
51794
|
Mutational, structural, and kinetic evidence for a dissociative mechanism in the GDP-mannose mannosyl ...
|
|
51795
|
Mutational, structural, and kinetic evidence for a dissociative mechanism in the GDP-mannose mannosyl ...
|
|
51796
|
Mutational, structural, and kinetic evidence for a dissociative mechanism in the GDP-mannose mannosyl ...
|
|
51797
|
Mutational, structural, and kinetic evidence for a dissociative mechanism in the GDP-mannose mannosyl ...
|
|
51798
|
Mutational, structural, and kinetic evidence for a dissociative mechanism in the GDP-mannose mannosyl ...
|
|
51799
|
Mutational, structural, and kinetic evidence for a dissociative mechanism in the GDP-mannose mannosyl ...
|
|
51800
|
Mutational, structural, and kinetic evidence for a dissociative mechanism in the GDP-mannose mannosyl ...
|
|
51801
|
Mutational, structural, and kinetic evidence for a dissociative mechanism in the GDP-mannose mannosyl ...
|
|
51802
|
Mutational, structural, and kinetic evidence for a dissociative mechanism in the GDP-mannose mannosyl ...
|
|
51803
|
Mutational, structural, and kinetic evidence for a dissociative mechanism in the GDP-mannose mannosyl ...
|
|
51804
|
Mutational, structural, and kinetic evidence for a dissociative mechanism in the GDP-mannose mannosyl ...
|
|
51805
|
Mutational, structural, and kinetic evidence for a dissociative mechanism in the GDP-mannose mannosyl ...
|
|
51806
|
Mutational, structural, and kinetic evidence for a dissociative mechanism in the GDP-mannose mannosyl ...
|
|
51807
|
Mutational, structural, and kinetic evidence for a dissociative mechanism in the GDP-mannose mannosyl ...
|
|
51808
|
Mutational, structural, and kinetic evidence for a dissociative mechanism in the GDP-mannose mannosyl ...
|
|
51809
|
Biochemical characterization of the human arsenite-stimulated ATPase (hASNA-I)
|
|
51810
|
Biochemical characterization of the human arsenite-stimulated ATPase (hASNA-I)
|
|
51811
|
Mutational, Kinetic, and NMR Studies of the Mechanism of E. coli GDP-Mannose Mannosyl Hydrolase, an Unusual ...
|
|
51812
|
Mutational, Kinetic, and NMR Studies of the Mechanism of E. coli GDP-Mannose Mannosyl Hydrolase, an Unusual ...
|
|
51813
|
Mutational, Kinetic, and NMR Studies of the Mechanism of E. coli GDP-Mannose Mannosyl Hydrolase, an Unusual ...
|
|
51814
|
Mutational, Kinetic, and NMR Studies of the Mechanism of E. coli GDP-Mannose Mannosyl Hydrolase, an Unusual ...
|
|
51815
|
Mutational, Kinetic, and NMR Studies of the Mechanism of E. coli GDP-Mannose Mannosyl Hydrolase, an Unusual ...
|
|
51816
|
Mutational, Kinetic, and NMR Studies of the Mechanism of E. coli GDP-Mannose Mannosyl Hydrolase, an Unusual ...
|
|
51817
|
Mutational, Kinetic, and NMR Studies of the Mechanism of E. coli GDP-Mannose Mannosyl Hydrolase, an Unusual ...
|
|
51818
|
Mutational, Kinetic, and NMR Studies of the Mechanism of E. coli GDP-Mannose Mannosyl Hydrolase, an Unusual ...
|
|
51819
|
Mutational, Kinetic, and NMR Studies of the Mechanism of E. coli GDP-Mannose Mannosyl Hydrolase, an Unusual ...
|
|
51820
|
Mutational, Kinetic, and NMR Studies of the Mechanism of E. coli GDP-Mannose Mannosyl Hydrolase, an Unusual ...
|
|
51821
|
Mutational, Kinetic, and NMR Studies of the Mechanism of E. coli GDP-Mannose Mannosyl Hydrolase, an Unusual ...
|
|
51822
|
Mutational, Kinetic, and NMR Studies of the Mechanism of E. coli GDP-Mannose Mannosyl Hydrolase, an Unusual ...
|
|
51823
|
Mutational, Kinetic, and NMR Studies of the Mechanism of E. coli GDP-Mannose Mannosyl Hydrolase, an Unusual ...
|
|
51824
|
Mutational, Kinetic, and NMR Studies of the Mechanism of E. coli GDP-Mannose Mannosyl Hydrolase, an Unusual ...
|
|
51825
|
Mutational, Kinetic, and NMR Studies of the Mechanism of E. coli GDP-Mannose Mannosyl Hydrolase, an Unusual ...
|
|
51826
|
Mutational, Kinetic, and NMR Studies of the Mechanism of E. coli GDP-Mannose Mannosyl Hydrolase, an Unusual ...
|
|
51827
|
Mutational, Kinetic, and NMR Studies of the Mechanism of E. coli GDP-Mannose Mannosyl Hydrolase, an Unusual ...
|
|
51828
|
Mutational, Kinetic, and NMR Studies of the Mechanism of E. coli GDP-Mannose Mannosyl Hydrolase, an Unusual ...
|
|
51829
|
Mutational, Kinetic, and NMR Studies of the Mechanism of E. coli GDP-Mannose Mannosyl Hydrolase, an Unusual ...
|
|
51830
|
Mutational, Kinetic, and NMR Studies of the Mechanism of E. coli GDP-Mannose Mannosyl Hydrolase, an Unusual ...
|
|
51831
|
Mutational, Kinetic, and NMR Studies of the Mechanism of E. coli GDP-Mannose Mannosyl Hydrolase, an Unusual ...
|
|
51832
|
Mutational, Kinetic, and NMR Studies of the Mechanism of E. coli GDP-Mannose Mannosyl Hydrolase, an Unusual ...
|
|
51833
|
Mutational, Kinetic, and NMR Studies of the Mechanism of E. coli GDP-Mannose Mannosyl Hydrolase, an Unusual ...
|
|
51834
|
Mutational, Kinetic, and NMR Studies of the Mechanism of E. coli GDP-Mannose Mannosyl Hydrolase, an Unusual ...
|
|
51835
|
Mutational, Kinetic, and NMR Studies of the Mechanism of E. coli GDP-Mannose Mannosyl Hydrolase, an Unusual ...
|
|
51836
|
Mutational, Kinetic, and NMR Studies of the Mechanism of E. coli GDP-Mannose Mannosyl Hydrolase, an Unusual ...
|
|
51837
|
Mutational, Kinetic, and NMR Studies of the Mechanism of E. coli GDP-Mannose Mannosyl Hydrolase, an Unusual ...
|
|
51838
|
Mutational, Kinetic, and NMR Studies of the Mechanism of E. coli GDP-Mannose Mannosyl Hydrolase, an Unusual ...
|
|
51839
|
Mutational, Kinetic, and NMR Studies of the Mechanism of E. coli GDP-Mannose Mannosyl Hydrolase, an Unusual ...
|
|
51840
|
Catalytic residues Lys197 and Arg199 of Bacillus subtilis phosphoribosyl diphosphate synthase. ...
|
|
51841
|
Catalytic residues Lys197 and Arg199 of Bacillus subtilis phosphoribosyl diphosphate synthase. ...
|
|
51842
|
Catalytic residues Lys197 and Arg199 of Bacillus subtilis phosphoribosyl diphosphate synthase. ...
|
|
51843
|
Catalytic residues Lys197 and Arg199 of Bacillus subtilis phosphoribosyl diphosphate synthase. ...
|
|
51844
|
Catalytic residues Lys197 and Arg199 of Bacillus subtilis phosphoribosyl diphosphate synthase. ...
|
|
51845
|
Catalytic residues Lys197 and Arg199 of Bacillus subtilis phosphoribosyl diphosphate synthase. ...
|
|
51846
|
Catalytic residues Lys197 and Arg199 of Bacillus subtilis phosphoribosyl diphosphate synthase. ...
|
|
51847
|
Catalytic residues Lys197 and Arg199 of Bacillus subtilis phosphoribosyl diphosphate synthase. ...
|
|
51848
|
Catalytic residues Lys197 and Arg199 of Bacillus subtilis phosphoribosyl diphosphate synthase. ...
|
|
51849
|
Catalytic residues Lys197 and Arg199 of Bacillus subtilis phosphoribosyl diphosphate synthase. ...
|
|
51850
|
Catalytic residues Lys197 and Arg199 of Bacillus subtilis phosphoribosyl diphosphate synthase. ...
|
|
51851
|
Catalytic residues Lys197 and Arg199 of Bacillus subtilis phosphoribosyl diphosphate synthase. ...
|
|
51852
|
Catalytic residues Lys197 and Arg199 of Bacillus subtilis phosphoribosyl diphosphate synthase. ...
|
|
51853
|
Catalytic residues Lys197 and Arg199 of Bacillus subtilis phosphoribosyl diphosphate synthase. ...
|
|
51854
|
Catalytic residues Lys197 and Arg199 of Bacillus subtilis phosphoribosyl diphosphate synthase. ...
|
|
51855
|
Catalytic residues Lys197 and Arg199 of Bacillus subtilis phosphoribosyl diphosphate synthase. ...
|
|
51856
|
Catalytic residues Lys197 and Arg199 of Bacillus subtilis phosphoribosyl diphosphate synthase. ...
|
|
51857
|
Catalytic residues Lys197 and Arg199 of Bacillus subtilis phosphoribosyl diphosphate synthase. ...
|
|
51858
|
Catalytic residues Lys197 and Arg199 of Bacillus subtilis phosphoribosyl diphosphate synthase. ...
|
|
51859
|
Catalytic residues Lys197 and Arg199 of Bacillus subtilis phosphoribosyl diphosphate synthase. ...
|
|
51860
|
Purification and characterization of an alcohol dehydrogenase from the Antarctic psychrophile Moraxella sp. ...
|
|
51861
|
Purification and characterization of an alcohol dehydrogenase from the Antarctic psychrophile Moraxella sp. ...
|
|
51862
|
Purification and characterization of an alcohol dehydrogenase from the Antarctic psychrophile Moraxella sp. ...
|
|
51863
|
Purification and characterization of an alcohol dehydrogenase from the Antarctic psychrophile Moraxella sp. ...
|
|
51864
|
Purification and characterization of an alcohol dehydrogenase from the Antarctic psychrophile Moraxella sp. ...
|
|
51865
|
Purification and characterization of an alcohol dehydrogenase from the Antarctic psychrophile Moraxella sp. ...
|
|
51866
|
Purification and characterization of an alcohol dehydrogenase from the Antarctic psychrophile Moraxella sp. ...
|
|
51867
|
Methionine metabolism in plants: chloroplasts are autonomous for de novo methionine synthesis and can import ...
|
|
51868
|
Methionine metabolism in plants: chloroplasts are autonomous for de novo methionine synthesis and can import ...
|
|
51869
|
Methionine metabolism in plants: chloroplasts are autonomous for de novo methionine synthesis and can import ...
|
|
51870
|
Methionine metabolism in plants: chloroplasts are autonomous for de novo methionine synthesis and can import ...
|
|
51871
|
Methionine metabolism in plants: chloroplasts are autonomous for de novo methionine synthesis and can import ...
|
|
51872
|
Construction of chimeric cytosolic fructose-1,6-bisphosphatases by insertion of a chloroplastic redox ...
|
|
51873
|
Construction of chimeric cytosolic fructose-1,6-bisphosphatases by insertion of a chloroplastic redox ...
|
|
51874
|
Construction of chimeric cytosolic fructose-1,6-bisphosphatases by insertion of a chloroplastic redox ...
|
|
51875
|
Construction of chimeric cytosolic fructose-1,6-bisphosphatases by insertion of a chloroplastic redox ...
|
|
51876
|
Construction of chimeric cytosolic fructose-1,6-bisphosphatases by insertion of a chloroplastic redox ...
|
|
51877
|
Construction of chimeric cytosolic fructose-1,6-bisphosphatases by insertion of a chloroplastic redox ...
|
|
51878
|
Construction of chimeric cytosolic fructose-1,6-bisphosphatases by insertion of a chloroplastic redox ...
|
|
51879
|
Construction of chimeric cytosolic fructose-1,6-bisphosphatases by insertion of a chloroplastic redox ...
|
|
51880
|
Construction of chimeric cytosolic fructose-1,6-bisphosphatases by insertion of a chloroplastic redox ...
|
|
51881
|
Construction of chimeric cytosolic fructose-1,6-bisphosphatases by insertion of a chloroplastic redox ...
|
|
51882
|
Construction of chimeric cytosolic fructose-1,6-bisphosphatases by insertion of a chloroplastic redox ...
|
|
51883
|
Cowchock syndrome is associated with a mutation in apoptosis-inducing factor.
|
|
51884
|
Cowchock syndrome is associated with a mutation in apoptosis-inducing factor.
|
|
51885
|
Cowchock syndrome is associated with a mutation in apoptosis-inducing factor.
|
|
51886
|
Cowchock syndrome is associated with a mutation in apoptosis-inducing factor.
|
|
51887
|
Cowchock syndrome is associated with a mutation in apoptosis-inducing factor.
|
|
51888
|
Cowchock syndrome is associated with a mutation in apoptosis-inducing factor.
|
|
51889
|
Cowchock syndrome is associated with a mutation in apoptosis-inducing factor.
|
|
51890
|
Cowchock syndrome is associated with a mutation in apoptosis-inducing factor.
|
|
51891
|
Cowchock syndrome is associated with a mutation in apoptosis-inducing factor.
|
|
51892
|
Cowchock syndrome is associated with a mutation in apoptosis-inducing factor.
|
|
51893
|
Cowchock syndrome is associated with a mutation in apoptosis-inducing factor.
|
|
51894
|
Cowchock syndrome is associated with a mutation in apoptosis-inducing factor.
|
|
51895
|
Convergent evolution of a 2-methylbutyryl-CoA dehydrogenase from isovaleryl-CoA dehydrogenase in Solanum ...
|
|
51896
|
Convergent evolution of a 2-methylbutyryl-CoA dehydrogenase from isovaleryl-CoA dehydrogenase in Solanum ...
|
|
51897
|
UMP kinase from the Gram-positive bacterium Bacillus subtilis is strongly dependent on GTP for optimal ...
|
|
51898
|
UMP kinase from the Gram-positive bacterium Bacillus subtilis is strongly dependent on GTP for optimal ...
|
|
51899
|
UMP kinase from the Gram-positive bacterium Bacillus subtilis is strongly dependent on GTP for optimal ...
|
|
51900
|
UMP kinase from the Gram-positive bacterium Bacillus subtilis is strongly dependent on GTP for optimal ...
|
|
51901
|
UMP kinase from the Gram-positive bacterium Bacillus subtilis is strongly dependent on GTP for optimal ...
|
|
51902
|
UMP kinase from the Gram-positive bacterium Bacillus subtilis is strongly dependent on GTP for optimal ...
|
|
51903
|
UMP kinase from the Gram-positive bacterium Bacillus subtilis is strongly dependent on GTP for optimal ...
|
|
51904
|
UMP kinase from the Gram-positive bacterium Bacillus subtilis is strongly dependent on GTP for optimal ...
|
|
51905
|
UMP kinase from the Gram-positive bacterium Bacillus subtilis is strongly dependent on GTP for optimal ...
|
|
51906
|
Molecular and kinetic characterization of two UDP-glucose pyrophosphorylases, products of distinct genes, from ...
|
|
51907
|
Molecular and kinetic characterization of two UDP-glucose pyrophosphorylases, products of distinct genes, from ...
|
|
51908
|
Molecular and kinetic characterization of two UDP-glucose pyrophosphorylases, products of distinct genes, from ...
|
|
51909
|
Molecular and kinetic characterization of two UDP-glucose pyrophosphorylases, products of distinct genes, from ...
|
|
51910
|
Molecular and kinetic characterization of two UDP-glucose pyrophosphorylases, products of distinct genes, from ...
|
|
51911
|
Molecular and kinetic characterization of two UDP-glucose pyrophosphorylases, products of distinct genes, from ...
|
|
51912
|
Molecular and kinetic characterization of two UDP-glucose pyrophosphorylases, products of distinct genes, from ...
|
|
51913
|
Molecular and kinetic characterization of two UDP-glucose pyrophosphorylases, products of distinct genes, from ...
|
|
51914
|
Molecular and kinetic characterization of two UDP-glucose pyrophosphorylases, products of distinct genes, from ...
|
|
51915
|
Molecular and kinetic characterization of two UDP-glucose pyrophosphorylases, products of distinct genes, from ...
|
|
51916
|
The antituberculosis drug ethionamide is activated by a flavoprotein monooxygenase
|
|
51917
|
Mechanistic aspects of the covalent flavoprotein dimethylglycine oxidase of Arthrobacter globiformis studied ...
|
|
51918
|
Mechanistic aspects of the covalent flavoprotein dimethylglycine oxidase of Arthrobacter globiformis studied ...
|
|
51919
|
Kinetic mechanism of Bacillus subtilis L-alanine dehydrogenase
|
|
51920
|
Kinetic mechanism of Bacillus subtilis L-alanine dehydrogenase
|
|
51921
|
Kinetic mechanism of Bacillus subtilis L-alanine dehydrogenase
|
|
51922
|
Kinetic mechanism of Bacillus subtilis L-alanine dehydrogenase
|
|
51923
|
Kinetic mechanism of Bacillus subtilis L-alanine dehydrogenase
|
|
51924
|
Kinetic mechanism of Bacillus subtilis L-alanine dehydrogenase
|
|
51925
|
Kinetic mechanism of Bacillus subtilis L-alanine dehydrogenase
|
|
51926
|
Kinetic mechanism of Bacillus subtilis L-alanine dehydrogenase
|
|
51927
|
Kinetic mechanism of Bacillus subtilis L-alanine dehydrogenase
|
|
51928
|
Kinetic mechanism of Bacillus subtilis L-alanine dehydrogenase
|
|
51929
|
Kinetic mechanism of Bacillus subtilis L-alanine dehydrogenase
|
|
51930
|
Kinetic mechanism of Bacillus subtilis L-alanine dehydrogenase
|
|
51931
|
Kinetic mechanism of Bacillus subtilis L-alanine dehydrogenase
|
|
51932
|
Kinetic mechanism of Bacillus subtilis L-alanine dehydrogenase
|
|
51933
|
Kinetic mechanism of Bacillus subtilis L-alanine dehydrogenase
|
|
51934
|
Kinetic mechanism of Bacillus subtilis L-alanine dehydrogenase
|
|
51935
|
Kinetic mechanism of Bacillus subtilis L-alanine dehydrogenase
|
|
51936
|
Kinetic mechanism of Bacillus subtilis L-alanine dehydrogenase
|
|
51937
|
Kinetic mechanism of Bacillus subtilis L-alanine dehydrogenase
|
|
51938
|
Kinetic mechanism of Bacillus subtilis L-alanine dehydrogenase
|
|
51939
|
Kinetic mechanism of Bacillus subtilis L-alanine dehydrogenase
|
|
51940
|
Kinetic mechanism of Bacillus subtilis L-alanine dehydrogenase
|
|
51941
|
Kinetic mechanism of Bacillus subtilis L-alanine dehydrogenase
|
|
51942
|
Kinetic mechanism of Bacillus subtilis L-alanine dehydrogenase
|
|
51943
|
Kinetic mechanism of Bacillus subtilis L-alanine dehydrogenase
|
|
51944
|
Kinetic mechanism of Bacillus subtilis L-alanine dehydrogenase
|
|
51945
|
Glycine oxidase from Bacillus subtilis: role of histidine 244 and methionine 261
|
|
51946
|
Glycine oxidase from Bacillus subtilis: role of histidine 244 and methionine 261
|
|
51947
|
Glycine oxidase from Bacillus subtilis: role of histidine 244 and methionine 261
|
|
51948
|
Glycine oxidase from Bacillus subtilis: role of histidine 244 and methionine 261
|
|
51949
|
Glycine oxidase from Bacillus subtilis: role of histidine 244 and methionine 261
|
|
51950
|
Glycine oxidase from Bacillus subtilis: role of histidine 244 and methionine 261
|
|
51951
|
Glycine oxidase from Bacillus subtilis: role of histidine 244 and methionine 261
|
|
51952
|
Glycine oxidase from Bacillus subtilis: role of histidine 244 and methionine 261
|
|
51953
|
Glycine oxidase from Bacillus subtilis: role of histidine 244 and methionine 261
|
|
51954
|
Glycine oxidase from Bacillus subtilis: role of histidine 244 and methionine 261
|
|
51955
|
Glycine oxidase from Bacillus subtilis: role of histidine 244 and methionine 261
|
|
51956
|
Glycine oxidase from Bacillus subtilis: role of histidine 244 and methionine 261
|
|
51957
|
Glycine oxidase from Bacillus subtilis: role of histidine 244 and methionine 261
|
|
51958
|
Glycine oxidase from Bacillus subtilis: role of histidine 244 and methionine 261
|
|
51959
|
Glycine oxidase from Bacillus subtilis: role of histidine 244 and methionine 261
|
|
51960
|
Glycine oxidase from Bacillus subtilis: role of histidine 244 and methionine 261
|
|
51961
|
Glycine oxidase from Bacillus subtilis: role of histidine 244 and methionine 261
|
|
51962
|
Glycine oxidase from Bacillus subtilis: role of histidine 244 and methionine 261
|
|
51963
|
Glycine oxidase from Bacillus subtilis: role of histidine 244 and methionine 261
|
|
51964
|
Glycine oxidase from Bacillus subtilis: role of histidine 244 and methionine 261
|
|
51965
|
Glycine oxidase from Bacillus subtilis: role of histidine 244 and methionine 261
|
|
51966
|
Glycine oxidase from Bacillus subtilis: role of histidine 244 and methionine 261
|
|
51967
|
Glycine oxidase from Bacillus subtilis: role of histidine 244 and methionine 261
|
|
51968
|
Glycine oxidase from Bacillus subtilis: role of histidine 244 and methionine 261
|
|
51969
|
Glycine oxidase from Bacillus subtilis: role of histidine 244 and methionine 261
|
|
51970
|
Glycine oxidase from Bacillus subtilis: role of histidine 244 and methionine 261
|
|
51971
|
Glycine oxidase from Bacillus subtilis: role of histidine 244 and methionine 261
|
|
51972
|
Glycine oxidase from Bacillus subtilis: role of histidine 244 and methionine 261
|
|
51973
|
Glycine oxidase from Bacillus subtilis: role of histidine 244 and methionine 261
|
|
51974
|
Glycine oxidase from Bacillus subtilis: role of histidine 244 and methionine 261
|
|
51975
|
Glycine oxidase from Bacillus subtilis: role of histidine 244 and methionine 261
|
|
51976
|
Glycine oxidase from Bacillus subtilis: role of histidine 244 and methionine 261
|
|
51977
|
Glycine oxidase from Bacillus subtilis: role of histidine 244 and methionine 261
|
|
51978
|
Glycine oxidase from Bacillus subtilis: role of histidine 244 and methionine 261
|
|
51979
|
Glycine oxidase from Bacillus subtilis: role of histidine 244 and methionine 261
|
|
51980
|
Glycine oxidase from Bacillus subtilis: role of histidine 244 and methionine 261
|
|
51981
|
Glycine oxidase from Bacillus subtilis: role of histidine 244 and methionine 261
|
|
51982
|
Glycine oxidase from Bacillus subtilis: role of histidine 244 and methionine 261
|
|
51983
|
Glycine oxidase from Bacillus subtilis: role of histidine 244 and methionine 261
|
|
51984
|
Glycine oxidase from Bacillus subtilis: role of histidine 244 and methionine 261
|
|
51985
|
Glycine oxidase from Bacillus subtilis: role of histidine 244 and methionine 261
|
|
51986
|
Glycine oxidase from Bacillus subtilis: role of histidine 244 and methionine 261
|
|
51987
|
Glycine oxidase from Bacillus subtilis: role of histidine 244 and methionine 261
|
|
51988
|
Glycine oxidase from Bacillus subtilis: role of histidine 244 and methionine 261
|
|
51989
|
Glycine oxidase from Bacillus subtilis: role of histidine 244 and methionine 261
|
|
51990
|
Glycine oxidase from Bacillus subtilis: role of histidine 244 and methionine 261
|
|
51991
|
Glycine oxidase from Bacillus subtilis: role of histidine 244 and methionine 261
|
|
51992
|
Glycine oxidase from Bacillus subtilis: role of histidine 244 and methionine 261
|
|
51993
|
Glycine oxidase from Bacillus subtilis: role of histidine 244 and methionine 261
|
|
51994
|
Glycine oxidase from Bacillus subtilis: role of histidine 244 and methionine 261
|
|
51995
|
Glycine oxidase from Bacillus subtilis: role of histidine 244 and methionine 261
|
|
51996
|
Glycine oxidase from Bacillus subtilis: role of histidine 244 and methionine 261
|
|
51997
|
Glycine oxidase from Bacillus subtilis: role of histidine 244 and methionine 261
|
|
51998
|
Glycine oxidase from Bacillus subtilis: role of histidine 244 and methionine 261
|
|
51999
|
Glycine oxidase from Bacillus subtilis: role of histidine 244 and methionine 261
|
|
52000
|
Glycine oxidase from Bacillus subtilis: role of histidine 244 and methionine 261
|




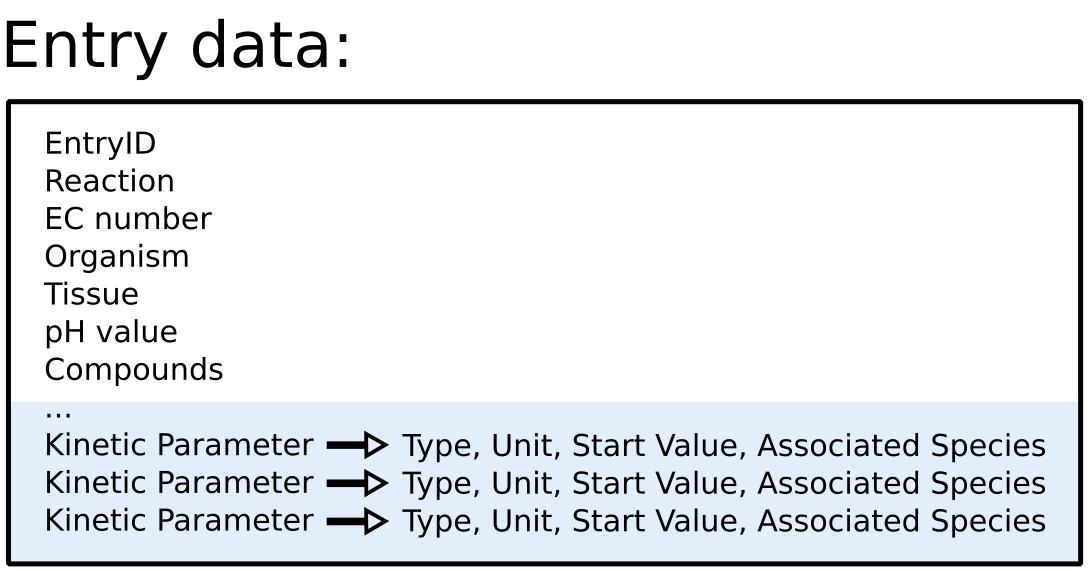
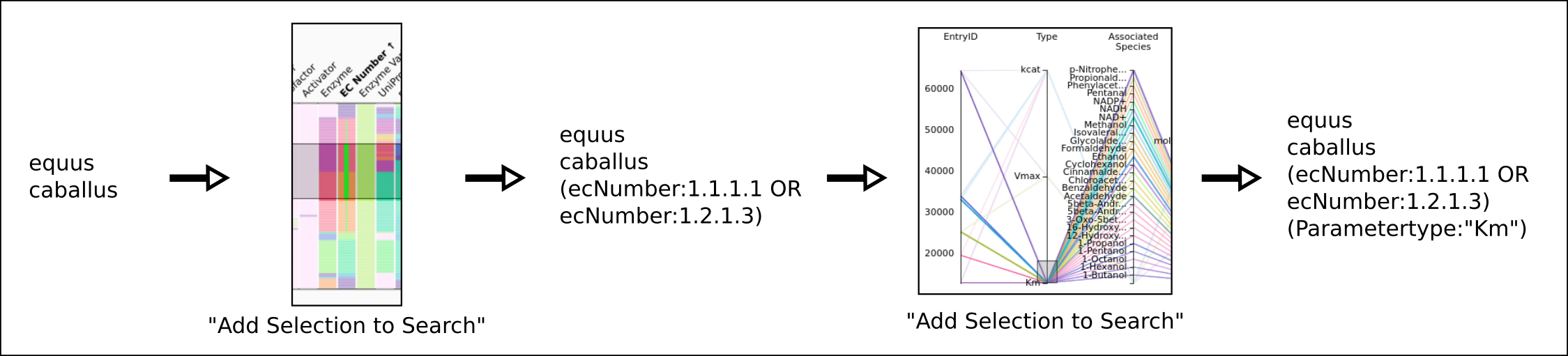 If the current search query is a refinement of the previous query "Use Previous Query (Go Back)" button can be used to perform the previous query again. Only one automatic step back is possible, but the query can be manually manipulated if desired.
If the current search query is a refinement of the previous query "Use Previous Query (Go Back)" button can be used to perform the previous query again. Only one automatic step back is possible, but the query can be manually manipulated if desired.