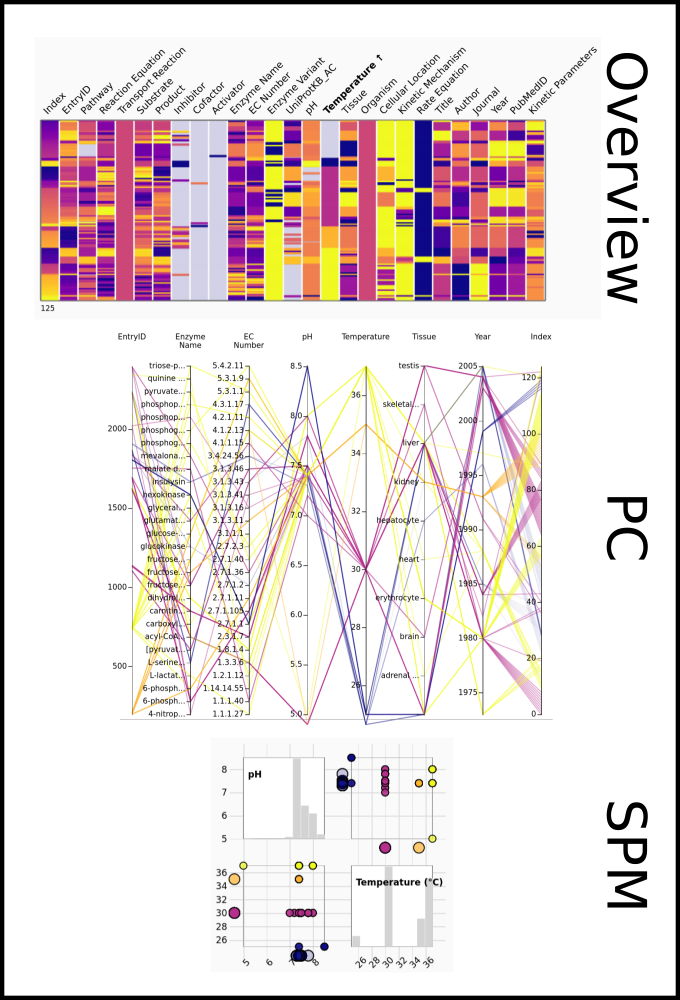
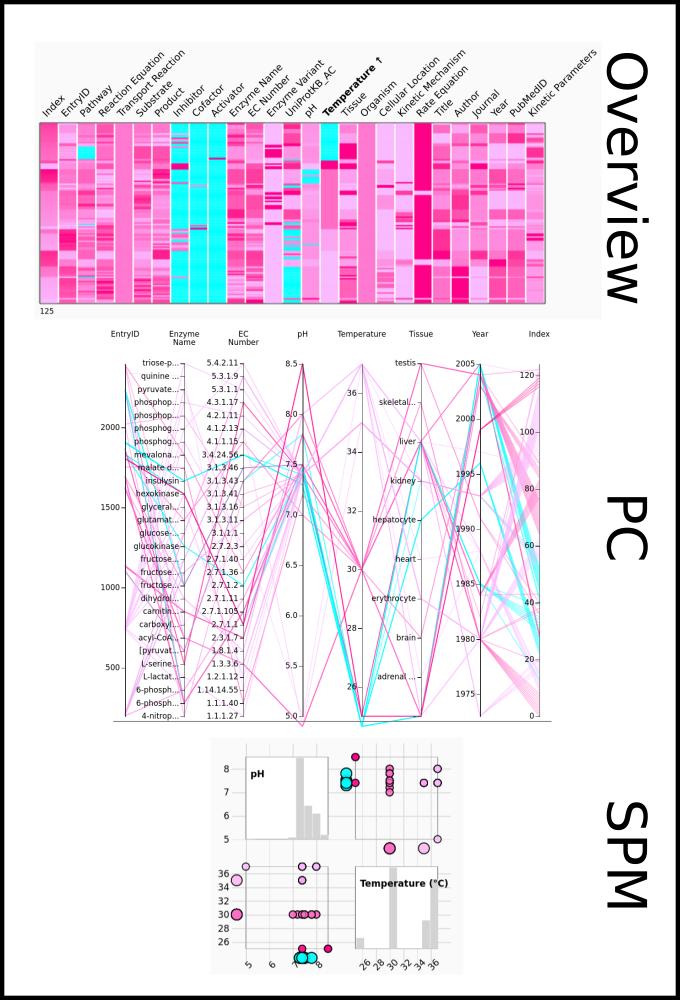
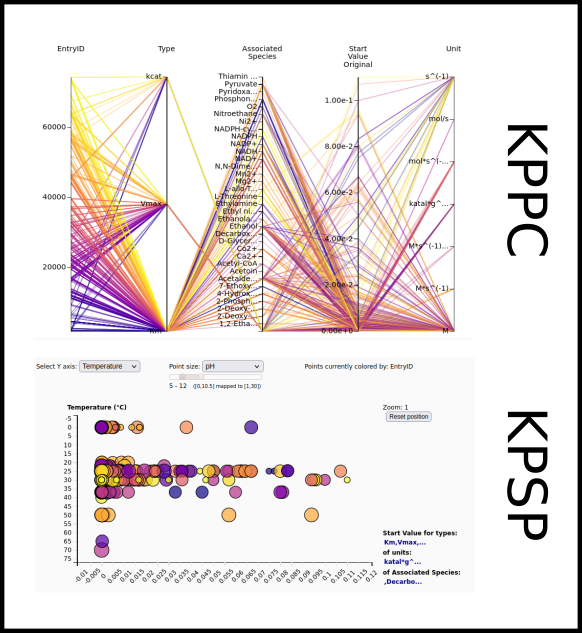
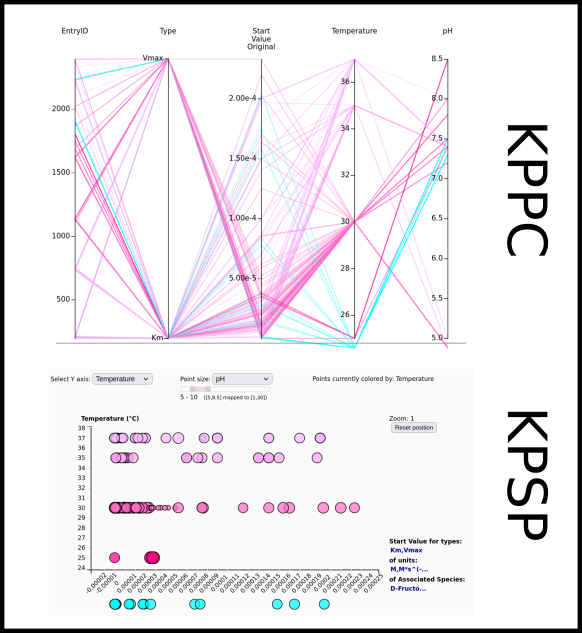
To improve SABIO-RK data interoperability, semantic markup was added to web pages as described and defined by
This structured information makes it easier to discover, collate and analyse our data.
|
17001
|
Allosteric kinetics of pyruvate kinase of Saccharomyces carlsbergensis
|
|
17002
|
Allosteric kinetics of pyruvate kinase of Saccharomyces carlsbergensis
|
|
17003
|
Allosteric kinetics of pyruvate kinase of Saccharomyces carlsbergensis
|
|
17004
|
Allosteric kinetics of pyruvate kinase of Saccharomyces carlsbergensis
|
|
17005
|
Molecular and kinetic comparison of the novel extended-spectrum beta-lactamases CTX-M-25 and CTX-M-26
|
|
17006
|
Molecular and kinetic comparison of the novel extended-spectrum beta-lactamases CTX-M-25 and CTX-M-26
|
|
17007
|
Molecular and kinetic comparison of the novel extended-spectrum beta-lactamases CTX-M-25 and CTX-M-26
|
|
17008
|
Molecular and kinetic comparison of the novel extended-spectrum beta-lactamases CTX-M-25 and CTX-M-26
|
|
17009
|
Molecular and kinetic comparison of the novel extended-spectrum beta-lactamases CTX-M-25 and CTX-M-26
|
|
17010
|
Molecular and kinetic comparison of the novel extended-spectrum beta-lactamases CTX-M-25 and CTX-M-26
|
|
17011
|
Molecular and kinetic comparison of the novel extended-spectrum beta-lactamases CTX-M-25 and CTX-M-26
|
|
17012
|
Molecular and kinetic comparison of the novel extended-spectrum beta-lactamases CTX-M-25 and CTX-M-26
|
|
17013
|
Molecular and kinetic comparison of the novel extended-spectrum beta-lactamases CTX-M-25 and CTX-M-26
|
|
17014
|
Molecular and kinetic comparison of the novel extended-spectrum beta-lactamases CTX-M-25 and CTX-M-26
|
|
17015
|
Molecular and kinetic comparison of the novel extended-spectrum beta-lactamases CTX-M-25 and CTX-M-26
|
|
17016
|
Molecular and kinetic comparison of the novel extended-spectrum beta-lactamases CTX-M-25 and CTX-M-26
|
|
17017
|
Molecular and kinetic comparison of the novel extended-spectrum beta-lactamases CTX-M-25 and CTX-M-26
|
|
17018
|
Molecular and kinetic comparison of the novel extended-spectrum beta-lactamases CTX-M-25 and CTX-M-26
|
|
17019
|
Molecular and kinetic comparison of the novel extended-spectrum beta-lactamases CTX-M-25 and CTX-M-26
|
|
17020
|
Molecular and kinetic comparison of the novel extended-spectrum beta-lactamases CTX-M-25 and CTX-M-26
|
|
17021
|
Molecular and kinetic comparison of the novel extended-spectrum beta-lactamases CTX-M-25 and CTX-M-26
|
|
17022
|
Molecular and kinetic comparison of the novel extended-spectrum beta-lactamases CTX-M-25 and CTX-M-26
|
|
17023
|
Molecular and kinetic comparison of the novel extended-spectrum beta-lactamases CTX-M-25 and CTX-M-26
|
|
17024
|
Molecular and kinetic comparison of the novel extended-spectrum beta-lactamases CTX-M-25 and CTX-M-26
|
|
17025
|
Molecular and kinetic comparison of the novel extended-spectrum beta-lactamases CTX-M-25 and CTX-M-26
|
|
17026
|
Molecular and kinetic comparison of the novel extended-spectrum beta-lactamases CTX-M-25 and CTX-M-26
|
|
17027
|
Role of arginase in sciatectomized muscle
|
|
17028
|
Role of arginase in sciatectomized muscle
|
|
17029
|
The active sites of fructose 6-phosphate,2-kinase: fructose-2, 6-bisphosphatase from rat testis. Roles of ...
|
|
17030
|
The active sites of fructose 6-phosphate,2-kinase: fructose-2, 6-bisphosphatase from rat testis. Roles of ...
|
|
17031
|
The active sites of fructose 6-phosphate,2-kinase: fructose-2, 6-bisphosphatase from rat testis. Roles of ...
|
|
17032
|
The active sites of fructose 6-phosphate,2-kinase: fructose-2, 6-bisphosphatase from rat testis. Roles of ...
|
|
17033
|
The active sites of fructose 6-phosphate,2-kinase: fructose-2, 6-bisphosphatase from rat testis. Roles of ...
|
|
17034
|
The active sites of fructose 6-phosphate,2-kinase: fructose-2, 6-bisphosphatase from rat testis. Roles of ...
|
|
17035
|
The active sites of fructose 6-phosphate,2-kinase: fructose-2, 6-bisphosphatase from rat testis. Roles of ...
|
|
17036
|
The active sites of fructose 6-phosphate,2-kinase: fructose-2, 6-bisphosphatase from rat testis. Roles of ...
|
|
17037
|
The active sites of fructose 6-phosphate,2-kinase: fructose-2, 6-bisphosphatase from rat testis. Roles of ...
|
|
17038
|
The active sites of fructose 6-phosphate,2-kinase: fructose-2, 6-bisphosphatase from rat testis. Roles of ...
|
|
17039
|
The active sites of fructose 6-phosphate,2-kinase: fructose-2, 6-bisphosphatase from rat testis. Roles of ...
|
|
17040
|
The active sites of fructose 6-phosphate,2-kinase: fructose-2, 6-bisphosphatase from rat testis. Roles of ...
|
|
17041
|
The active sites of fructose 6-phosphate,2-kinase: fructose-2, 6-bisphosphatase from rat testis. Roles of ...
|
|
17042
|
The active sites of fructose 6-phosphate,2-kinase: fructose-2, 6-bisphosphatase from rat testis. Roles of ...
|
|
17043
|
The active sites of fructose 6-phosphate,2-kinase: fructose-2, 6-bisphosphatase from rat testis. Roles of ...
|
|
17044
|
The active sites of fructose 6-phosphate,2-kinase: fructose-2, 6-bisphosphatase from rat testis. Roles of ...
|
|
17045
|
The active sites of fructose 6-phosphate,2-kinase: fructose-2, 6-bisphosphatase from rat testis. Roles of ...
|
|
17046
|
The active sites of fructose 6-phosphate,2-kinase: fructose-2, 6-bisphosphatase from rat testis. Roles of ...
|
|
17047
|
The active sites of fructose 6-phosphate,2-kinase: fructose-2, 6-bisphosphatase from rat testis. Roles of ...
|
|
17048
|
The active sites of fructose 6-phosphate,2-kinase: fructose-2, 6-bisphosphatase from rat testis. Roles of ...
|
|
17049
|
The active sites of fructose 6-phosphate,2-kinase: fructose-2, 6-bisphosphatase from rat testis. Roles of ...
|
|
17050
|
The active sites of fructose 6-phosphate,2-kinase: fructose-2, 6-bisphosphatase from rat testis. Roles of ...
|
|
17051
|
The active sites of fructose 6-phosphate,2-kinase: fructose-2, 6-bisphosphatase from rat testis. Roles of ...
|
|
17052
|
The active sites of fructose 6-phosphate,2-kinase: fructose-2, 6-bisphosphatase from rat testis. Roles of ...
|
|
17053
|
The active sites of fructose 6-phosphate,2-kinase: fructose-2, 6-bisphosphatase from rat testis. Roles of ...
|
|
17054
|
The active sites of fructose 6-phosphate,2-kinase: fructose-2, 6-bisphosphatase from rat testis. Roles of ...
|
|
17055
|
The active sites of fructose 6-phosphate,2-kinase: fructose-2, 6-bisphosphatase from rat testis. Roles of ...
|
|
17056
|
The active sites of fructose 6-phosphate,2-kinase: fructose-2, 6-bisphosphatase from rat testis. Roles of ...
|
|
17057
|
The active sites of fructose 6-phosphate,2-kinase: fructose-2, 6-bisphosphatase from rat testis. Roles of ...
|
|
17058
|
The active sites of fructose 6-phosphate,2-kinase: fructose-2, 6-bisphosphatase from rat testis. Roles of ...
|
|
17059
|
The active sites of fructose 6-phosphate,2-kinase: fructose-2, 6-bisphosphatase from rat testis. Roles of ...
|
|
17060
|
The active sites of fructose 6-phosphate,2-kinase: fructose-2, 6-bisphosphatase from rat testis. Roles of ...
|
|
17061
|
The active sites of fructose 6-phosphate,2-kinase: fructose-2, 6-bisphosphatase from rat testis. Roles of ...
|
|
17062
|
The active sites of fructose 6-phosphate,2-kinase: fructose-2, 6-bisphosphatase from rat testis. Roles of ...
|
|
17063
|
The active sites of fructose 6-phosphate,2-kinase: fructose-2, 6-bisphosphatase from rat testis. Roles of ...
|
|
17064
|
The active sites of fructose 6-phosphate,2-kinase: fructose-2, 6-bisphosphatase from rat testis. Roles of ...
|
|
17065
|
The active sites of fructose 6-phosphate,2-kinase: fructose-2, 6-bisphosphatase from rat testis. Roles of ...
|
|
17066
|
The active sites of fructose 6-phosphate,2-kinase: fructose-2, 6-bisphosphatase from rat testis. Roles of ...
|
|
17067
|
The active sites of fructose 6-phosphate,2-kinase: fructose-2, 6-bisphosphatase from rat testis. Roles of ...
|
|
17068
|
Rat kidney L-alpha-hydroxy acid oxidase: isolation of enzyme with one flavine coenzyme per two subunits
|
|
17069
|
Rat kidney L-alpha-hydroxy acid oxidase: isolation of enzyme with one flavine coenzyme per two subunits
|
|
17070
|
Rat kidney L-alpha-hydroxy acid oxidase: isolation of enzyme with one flavine coenzyme per two subunits
|
|
17071
|
Rat kidney L-alpha-hydroxy acid oxidase: isolation of enzyme with one flavine coenzyme per two subunits
|
|
17072
|
Rat kidney L-alpha-hydroxy acid oxidase: isolation of enzyme with one flavine coenzyme per two subunits
|
|
17073
|
Rat kidney L-alpha-hydroxy acid oxidase: isolation of enzyme with one flavine coenzyme per two subunits
|
|
17074
|
Rat kidney L-alpha-hydroxy acid oxidase: isolation of enzyme with one flavine coenzyme per two subunits
|
|
17075
|
Rat kidney L-alpha-hydroxy acid oxidase: isolation of enzyme with one flavine coenzyme per two subunits
|
|
17076
|
Rat kidney L-alpha-hydroxy acid oxidase: isolation of enzyme with one flavine coenzyme per two subunits
|
|
17077
|
Rat kidney L-alpha-hydroxy acid oxidase: isolation of enzyme with one flavine coenzyme per two subunits
|
|
17078
|
Rat kidney L-alpha-hydroxy acid oxidase: isolation of enzyme with one flavine coenzyme per two subunits
|
|
17079
|
Rat kidney L-alpha-hydroxy acid oxidase: isolation of enzyme with one flavine coenzyme per two subunits
|
|
17080
|
Rat kidney L-alpha-hydroxy acid oxidase: isolation of enzyme with one flavine coenzyme per two subunits
|
|
17081
|
Identification and isolation on a large scale of guanylate kinase from human erythrocytes. Effects of ...
|
|
17082
|
Identification and isolation on a large scale of guanylate kinase from human erythrocytes. Effects of ...
|
|
17083
|
Identification and isolation on a large scale of guanylate kinase from human erythrocytes. Effects of ...
|
|
17084
|
Identification and isolation on a large scale of guanylate kinase from human erythrocytes. Effects of ...
|
|
17085
|
Identification and isolation on a large scale of guanylate kinase from human erythrocytes. Effects of ...
|
|
17086
|
Identification and isolation on a large scale of guanylate kinase from human erythrocytes. Effects of ...
|
|
17087
|
Inositol polyphosphate multikinase is a nuclear PI3-kinase with transcriptional regulatory activity
|
|
17088
|
Inositol polyphosphate multikinase is a nuclear PI3-kinase with transcriptional regulatory activity
|
|
17089
|
Two forms of NAD-dependent D-mandelate dehydrogenase in Enterococcus faecalis IAM 10071
|
|
17090
|
Two forms of NAD-dependent D-mandelate dehydrogenase in Enterococcus faecalis IAM 10071
|
|
17091
|
Two forms of NAD-dependent D-mandelate dehydrogenase in Enterococcus faecalis IAM 10071
|
|
17092
|
Two forms of NAD-dependent D-mandelate dehydrogenase in Enterococcus faecalis IAM 10071
|
|
17093
|
Two forms of NAD-dependent D-mandelate dehydrogenase in Enterococcus faecalis IAM 10071
|
|
17094
|
Two forms of NAD-dependent D-mandelate dehydrogenase in Enterococcus faecalis IAM 10071
|
|
17095
|
Two forms of NAD-dependent D-mandelate dehydrogenase in Enterococcus faecalis IAM 10071
|
|
17096
|
Two forms of NAD-dependent D-mandelate dehydrogenase in Enterococcus faecalis IAM 10071
|
|
17097
|
Two forms of NAD-dependent D-mandelate dehydrogenase in Enterococcus faecalis IAM 10071
|
|
17098
|
Two forms of NAD-dependent D-mandelate dehydrogenase in Enterococcus faecalis IAM 10071
|
|
17099
|
Two forms of NAD-dependent D-mandelate dehydrogenase in Enterococcus faecalis IAM 10071
|
|
17100
|
Two forms of NAD-dependent D-mandelate dehydrogenase in Enterococcus faecalis IAM 10071
|
|
17101
|
Two forms of NAD-dependent D-mandelate dehydrogenase in Enterococcus faecalis IAM 10071
|
|
17102
|
Two forms of NAD-dependent D-mandelate dehydrogenase in Enterococcus faecalis IAM 10071
|
|
17103
|
Two forms of NAD-dependent D-mandelate dehydrogenase in Enterococcus faecalis IAM 10071
|
|
17104
|
Two forms of NAD-dependent D-mandelate dehydrogenase in Enterococcus faecalis IAM 10071
|
|
17105
|
Two forms of NAD-dependent D-mandelate dehydrogenase in Enterococcus faecalis IAM 10071
|
|
17106
|
Two forms of NAD-dependent D-mandelate dehydrogenase in Enterococcus faecalis IAM 10071
|
|
17107
|
Purification and Characterization of a Tripeptidase from Lactococcus lactis subsp. cremoris Wg2
|
|
17108
|
Identification of the GTP binding site of human glutamate dehydrogenase by cassette mutagenesis and ...
|
|
17109
|
Identification of the GTP binding site of human glutamate dehydrogenase by cassette mutagenesis and ...
|
|
17110
|
Identification of the GTP binding site of human glutamate dehydrogenase by cassette mutagenesis and ...
|
|
17111
|
Identification of the GTP binding site of human glutamate dehydrogenase by cassette mutagenesis and ...
|
|
17112
|
Identification of the GTP binding site of human glutamate dehydrogenase by cassette mutagenesis and ...
|
|
17113
|
Identification of the GTP binding site of human glutamate dehydrogenase by cassette mutagenesis and ...
|
|
17114
|
Identification of the GTP binding site of human glutamate dehydrogenase by cassette mutagenesis and ...
|
|
17115
|
Identification of the GTP binding site of human glutamate dehydrogenase by cassette mutagenesis and ...
|
|
17116
|
Identification of the GTP binding site of human glutamate dehydrogenase by cassette mutagenesis and ...
|
|
17117
|
Identification of the GTP binding site of human glutamate dehydrogenase by cassette mutagenesis and ...
|
|
17118
|
Identification of the GTP binding site of human glutamate dehydrogenase by cassette mutagenesis and ...
|
|
17119
|
Phosphoglycerate kinase B from ram testis. Purification, characterisation and comparison with the muscle ...
|
|
17120
|
Phosphoglycerate kinase B from ram testis. Purification, characterisation and comparison with the muscle ...
|
|
17121
|
Phosphoglycerate kinase B from ram testis. Purification, characterisation and comparison with the muscle ...
|
|
17122
|
Phosphoglycerate kinase B from ram testis. Purification, characterisation and comparison with the muscle ...
|
|
17123
|
Phosphoglycerate kinase B from ram testis. Purification, characterisation and comparison with the muscle ...
|
|
17124
|
Phosphoglycerate kinase B from ram testis. Purification, characterisation and comparison with the muscle ...
|
|
17125
|
Substitution of Asp for Asn at position 132 in the active site of TEM beta-lactamase. Activity toward ...
|
|
17126
|
Substitution of Asp for Asn at position 132 in the active site of TEM beta-lactamase. Activity toward ...
|
|
17127
|
Substitution of Asp for Asn at position 132 in the active site of TEM beta-lactamase. Activity toward ...
|
|
17128
|
Substitution of Asp for Asn at position 132 in the active site of TEM beta-lactamase. Activity toward ...
|
|
17129
|
Substitution of Asp for Asn at position 132 in the active site of TEM beta-lactamase. Activity toward ...
|
|
17130
|
Substitution of Asp for Asn at position 132 in the active site of TEM beta-lactamase. Activity toward ...
|
|
17131
|
Substitution of Asp for Asn at position 132 in the active site of TEM beta-lactamase. Activity toward ...
|
|
17132
|
Substitution of Asp for Asn at position 132 in the active site of TEM beta-lactamase. Activity toward ...
|
|
17133
|
Chemical modification and site-directed mutagenesis studies of rat 3-hydroxyisobutyrate dehydrogenase
|
|
17134
|
Chemical modification and site-directed mutagenesis studies of rat 3-hydroxyisobutyrate dehydrogenase
|
|
17135
|
Chemical modification and site-directed mutagenesis studies of rat 3-hydroxyisobutyrate dehydrogenase
|
|
17136
|
Chemical modification and site-directed mutagenesis studies of rat 3-hydroxyisobutyrate dehydrogenase
|
|
17137
|
Chemical modification and site-directed mutagenesis studies of rat 3-hydroxyisobutyrate dehydrogenase
|
|
17138
|
Chemical modification and site-directed mutagenesis studies of rat 3-hydroxyisobutyrate dehydrogenase
|
|
17139
|
Chemical modification and site-directed mutagenesis studies of rat 3-hydroxyisobutyrate dehydrogenase
|
|
17140
|
Chemical modification and site-directed mutagenesis studies of rat 3-hydroxyisobutyrate dehydrogenase
|
|
17141
|
Chemical modification and site-directed mutagenesis studies of rat 3-hydroxyisobutyrate dehydrogenase
|
|
17142
|
A study of the characteristics of hepatic iodothyronine 5'-monodeiodinase in various vertebrate species
|
|
17143
|
A study of the characteristics of hepatic iodothyronine 5'-monodeiodinase in various vertebrate species
|
|
17144
|
A study of the characteristics of hepatic iodothyronine 5'-monodeiodinase in various vertebrate species
|
|
17145
|
A study of the characteristics of hepatic iodothyronine 5'-monodeiodinase in various vertebrate species
|
|
17146
|
A study of the characteristics of hepatic iodothyronine 5'-monodeiodinase in various vertebrate species
|
|
17147
|
A study of the characteristics of hepatic iodothyronine 5'-monodeiodinase in various vertebrate species
|
|
17148
|
A study of the characteristics of hepatic iodothyronine 5'-monodeiodinase in various vertebrate species
|
|
17149
|
A study of the characteristics of hepatic iodothyronine 5'-monodeiodinase in various vertebrate species
|
|
17150
|
A study of the characteristics of hepatic iodothyronine 5'-monodeiodinase in various vertebrate species
|
|
17151
|
A study of the characteristics of hepatic iodothyronine 5'-monodeiodinase in various vertebrate species
|
|
17152
|
A study of the characteristics of hepatic iodothyronine 5'-monodeiodinase in various vertebrate species
|
|
17153
|
A study of the characteristics of hepatic iodothyronine 5'-monodeiodinase in various vertebrate species
|
|
17154
|
A study of the characteristics of hepatic iodothyronine 5'-monodeiodinase in various vertebrate species
|
|
17155
|
A study of the characteristics of hepatic iodothyronine 5'-monodeiodinase in various vertebrate species
|
|
17156
|
A study of the characteristics of hepatic iodothyronine 5'-monodeiodinase in various vertebrate species
|
|
17157
|
A study of the characteristics of hepatic iodothyronine 5'-monodeiodinase in various vertebrate species
|
|
17158
|
Inhibition of rat liver arginase by an intermediate in NO biosynthesis, NG-hydroxy-L-arginine: implications ...
|
|
17159
|
Inhibition of rat liver arginase by an intermediate in NO biosynthesis, NG-hydroxy-L-arginine: implications ...
|
|
17160
|
Inhibition of rat liver arginase by an intermediate in NO biosynthesis, NG-hydroxy-L-arginine: implications ...
|
|
17161
|
Inhibition of rat liver arginase by an intermediate in NO biosynthesis, NG-hydroxy-L-arginine: implications ...
|
|
17162
|
Inhibition of rat liver arginase by an intermediate in NO biosynthesis, NG-hydroxy-L-arginine: implications ...
|
|
17163
|
Inhibition of rat liver arginase by an intermediate in NO biosynthesis, NG-hydroxy-L-arginine: implications ...
|
|
17164
|
Inhibition of rat liver arginase by an intermediate in NO biosynthesis, NG-hydroxy-L-arginine: implications ...
|
|
17165
|
Inhibition of rat liver arginase by an intermediate in NO biosynthesis, NG-hydroxy-L-arginine: implications ...
|
|
17166
|
Inhibition of rat liver arginase by an intermediate in NO biosynthesis, NG-hydroxy-L-arginine: implications ...
|
|
17167
|
Inhibition of rat liver arginase by an intermediate in NO biosynthesis, NG-hydroxy-L-arginine: implications ...
|
|
17168
|
Purification and properties of liver arginase from teleostean fish Clarias batrachus (L.)
|
|
17169
|
Purification and properties of liver arginase from teleostean fish Clarias batrachus (L.)
|
|
17170
|
Purification and properties of liver arginase from teleostean fish Clarias batrachus (L.)
|
|
17171
|
Purification and properties of liver arginase from teleostean fish Clarias batrachus (L.)
|
|
17172
|
Purification and properties of liver arginase from teleostean fish Clarias batrachus (L.)
|
|
17173
|
Purification and properties of liver arginase from teleostean fish Clarias batrachus (L.)
|
|
17174
|
Purification and properties of liver arginase from teleostean fish Clarias batrachus (L.)
|
|
17175
|
Purification and properties of liver arginase from teleostean fish Clarias batrachus (L.)
|
|
17176
|
Purification and properties of liver arginase from teleostean fish Clarias batrachus (L.)
|
|
17177
|
Purification and properties of liver arginase from teleostean fish Clarias batrachus (L.)
|
|
17178
|
Purification and properties of liver arginase from teleostean fish Clarias batrachus (L.)
|
|
17179
|
Purification and properties of liver arginase from teleostean fish Clarias batrachus (L.)
|
|
17180
|
Purification and properties of liver arginase from teleostean fish Clarias batrachus (L.)
|
|
17181
|
An arginine regulated gamma-guanidobutyrate ureahydrolase from tench liver (Tinca tinca L.)
|
|
17182
|
An arginine regulated gamma-guanidobutyrate ureahydrolase from tench liver (Tinca tinca L.)
|
|
17183
|
An arginine regulated gamma-guanidobutyrate ureahydrolase from tench liver (Tinca tinca L.)
|
|
17184
|
An arginine regulated gamma-guanidobutyrate ureahydrolase from tench liver (Tinca tinca L.)
|
|
17185
|
An arginine regulated gamma-guanidobutyrate ureahydrolase from tench liver (Tinca tinca L.)
|
|
17186
|
Functional significance of a natural allelic variant of human carbonyl reductase 3 (CBR3)
|
|
17187
|
Functional significance of a natural allelic variant of human carbonyl reductase 3 (CBR3)
|
|
17188
|
Functional significance of a natural allelic variant of human carbonyl reductase 3 (CBR3)
|
|
17189
|
Functional significance of a natural allelic variant of human carbonyl reductase 3 (CBR3)
|
|
17190
|
Metabolism of melatonin by human cytochromes p450
|
|
17191
|
Metabolism of melatonin by human cytochromes p450
|
|
17192
|
Metabolism of melatonin by human cytochromes p450
|
|
17193
|
Metabolism of melatonin by human cytochromes p450
|
|
17194
|
A novel microperoxidase activity: methyl viologen-linked nitrite reducing activity of microperoxidase
|
|
17195
|
A novel microperoxidase activity: methyl viologen-linked nitrite reducing activity of microperoxidase
|
|
17196
|
A novel microperoxidase activity: methyl viologen-linked nitrite reducing activity of microperoxidase
|
|
17197
|
A novel microperoxidase activity: methyl viologen-linked nitrite reducing activity of microperoxidase
|
|
17198
|
A novel microperoxidase activity: methyl viologen-linked nitrite reducing activity of microperoxidase
|
|
17199
|
A novel microperoxidase activity: methyl viologen-linked nitrite reducing activity of microperoxidase
|
|
17200
|
Functional characterization of single nucleotide polymorphisms with amino acid substitution in CYP1A2, CYP2A6, ...
|
|
17201
|
Functional characterization of single nucleotide polymorphisms with amino acid substitution in CYP1A2, CYP2A6, ...
|
|
17202
|
Functional characterization of single nucleotide polymorphisms with amino acid substitution in CYP1A2, CYP2A6, ...
|
|
17203
|
Functional characterization of single nucleotide polymorphisms with amino acid substitution in CYP1A2, CYP2A6, ...
|
|
17204
|
Functional characterization of single nucleotide polymorphisms with amino acid substitution in CYP1A2, CYP2A6, ...
|
|
17205
|
Functional characterization of single nucleotide polymorphisms with amino acid substitution in CYP1A2, CYP2A6, ...
|
|
17206
|
Functional characterization of single nucleotide polymorphisms with amino acid substitution in CYP1A2, CYP2A6, ...
|
|
17207
|
Functional characterization of single nucleotide polymorphisms with amino acid substitution in CYP1A2, CYP2A6, ...
|
|
17208
|
Functional characterization of single nucleotide polymorphisms with amino acid substitution in CYP1A2, CYP2A6, ...
|
|
17209
|
CMP kinase from Escherichia coli is structurally related to other nucleoside monophosphate kinases
|
|
17210
|
CMP kinase from Escherichia coli is structurally related to other nucleoside monophosphate kinases
|
|
17211
|
CMP kinase from Escherichia coli is structurally related to other nucleoside monophosphate kinases
|
|
17212
|
CMP kinase from Escherichia coli is structurally related to other nucleoside monophosphate kinases
|
|
17213
|
CMP kinase from Escherichia coli is structurally related to other nucleoside monophosphate kinases
|
|
17214
|
CMP kinase from Escherichia coli is structurally related to other nucleoside monophosphate kinases
|
|
17215
|
CMP kinase from Escherichia coli is structurally related to other nucleoside monophosphate kinases
|
|
17216
|
CMP kinase from Escherichia coli is structurally related to other nucleoside monophosphate kinases
|
|
17217
|
CMP kinase from Escherichia coli is structurally related to other nucleoside monophosphate kinases
|
|
17218
|
Hydrolysis of diadenosine polyphosphates by nucleotide pyrophosphatases/phosphodiesterases
|
|
17219
|
Hydrolysis of diadenosine polyphosphates by nucleotide pyrophosphatases/phosphodiesterases
|
|
17220
|
Hydrolysis of diadenosine polyphosphates by nucleotide pyrophosphatases/phosphodiesterases
|
|
17221
|
A novel non-catalytic mechanism employed by the C-terminal Src-homologous kinase to inhibit Src-family kinase ...
|
|
17222
|
Probing the specificity of the subclass B3 FEZ-1 metallo-beta-lactamase by site-directed mutagenesis
|
|
17223
|
Probing the specificity of the subclass B3 FEZ-1 metallo-beta-lactamase by site-directed mutagenesis
|
|
17224
|
Probing the specificity of the subclass B3 FEZ-1 metallo-beta-lactamase by site-directed mutagenesis
|
|
17225
|
Probing the specificity of the subclass B3 FEZ-1 metallo-beta-lactamase by site-directed mutagenesis
|
|
17226
|
Probing the specificity of the subclass B3 FEZ-1 metallo-beta-lactamase by site-directed mutagenesis
|
|
17227
|
Probing the specificity of the subclass B3 FEZ-1 metallo-beta-lactamase by site-directed mutagenesis
|
|
17228
|
Probing the specificity of the subclass B3 FEZ-1 metallo-beta-lactamase by site-directed mutagenesis
|
|
17229
|
Probing the specificity of the subclass B3 FEZ-1 metallo-beta-lactamase by site-directed mutagenesis
|
|
17230
|
Probing the specificity of the subclass B3 FEZ-1 metallo-beta-lactamase by site-directed mutagenesis
|
|
17231
|
Probing the specificity of the subclass B3 FEZ-1 metallo-beta-lactamase by site-directed mutagenesis
|
|
17232
|
Probing the specificity of the subclass B3 FEZ-1 metallo-beta-lactamase by site-directed mutagenesis
|
|
17233
|
Probing the specificity of the subclass B3 FEZ-1 metallo-beta-lactamase by site-directed mutagenesis
|
|
17234
|
Probing the specificity of the subclass B3 FEZ-1 metallo-beta-lactamase by site-directed mutagenesis
|
|
17235
|
Probing the specificity of the subclass B3 FEZ-1 metallo-beta-lactamase by site-directed mutagenesis
|
|
17236
|
Probing the specificity of the subclass B3 FEZ-1 metallo-beta-lactamase by site-directed mutagenesis
|
|
17237
|
Probing the specificity of the subclass B3 FEZ-1 metallo-beta-lactamase by site-directed mutagenesis
|
|
17238
|
Probing the specificity of the subclass B3 FEZ-1 metallo-beta-lactamase by site-directed mutagenesis
|
|
17239
|
Probing the specificity of the subclass B3 FEZ-1 metallo-beta-lactamase by site-directed mutagenesis
|
|
17240
|
Probing the specificity of the subclass B3 FEZ-1 metallo-beta-lactamase by site-directed mutagenesis
|
|
17241
|
Probing the specificity of the subclass B3 FEZ-1 metallo-beta-lactamase by site-directed mutagenesis
|
|
17242
|
Probing the specificity of the subclass B3 FEZ-1 metallo-beta-lactamase by site-directed mutagenesis
|
|
17243
|
Probing the specificity of the subclass B3 FEZ-1 metallo-beta-lactamase by site-directed mutagenesis
|
|
17244
|
Probing the specificity of the subclass B3 FEZ-1 metallo-beta-lactamase by site-directed mutagenesis
|
|
17245
|
Probing the specificity of the subclass B3 FEZ-1 metallo-beta-lactamase by site-directed mutagenesis
|
|
17246
|
Probing the specificity of the subclass B3 FEZ-1 metallo-beta-lactamase by site-directed mutagenesis
|
|
17247
|
Probing the specificity of the subclass B3 FEZ-1 metallo-beta-lactamase by site-directed mutagenesis
|
|
17248
|
Probing the specificity of the subclass B3 FEZ-1 metallo-beta-lactamase by site-directed mutagenesis
|
|
17249
|
Probing the specificity of the subclass B3 FEZ-1 metallo-beta-lactamase by site-directed mutagenesis
|
|
17250
|
Probing the specificity of the subclass B3 FEZ-1 metallo-beta-lactamase by site-directed mutagenesis
|
|
17251
|
Probing the specificity of the subclass B3 FEZ-1 metallo-beta-lactamase by site-directed mutagenesis
|
|
17252
|
Probing the specificity of the subclass B3 FEZ-1 metallo-beta-lactamase by site-directed mutagenesis
|
|
17253
|
Probing the specificity of the subclass B3 FEZ-1 metallo-beta-lactamase by site-directed mutagenesis
|
|
17254
|
Probing the specificity of the subclass B3 FEZ-1 metallo-beta-lactamase by site-directed mutagenesis
|
|
17255
|
Probing the specificity of the subclass B3 FEZ-1 metallo-beta-lactamase by site-directed mutagenesis
|
|
17256
|
Probing the specificity of the subclass B3 FEZ-1 metallo-beta-lactamase by site-directed mutagenesis
|
|
17257
|
Probing the specificity of the subclass B3 FEZ-1 metallo-beta-lactamase by site-directed mutagenesis
|
|
17258
|
Probing the specificity of the subclass B3 FEZ-1 metallo-beta-lactamase by site-directed mutagenesis
|
|
17259
|
Probing the specificity of the subclass B3 FEZ-1 metallo-beta-lactamase by site-directed mutagenesis
|
|
17260
|
Probing the specificity of the subclass B3 FEZ-1 metallo-beta-lactamase by site-directed mutagenesis
|
|
17261
|
Probing the specificity of the subclass B3 FEZ-1 metallo-beta-lactamase by site-directed mutagenesis
|
|
17262
|
Probing the specificity of the subclass B3 FEZ-1 metallo-beta-lactamase by site-directed mutagenesis
|
|
17263
|
Probing the specificity of the subclass B3 FEZ-1 metallo-beta-lactamase by site-directed mutagenesis
|
|
17264
|
Probing the specificity of the subclass B3 FEZ-1 metallo-beta-lactamase by site-directed mutagenesis
|
|
17265
|
Probing the specificity of the subclass B3 FEZ-1 metallo-beta-lactamase by site-directed mutagenesis
|
|
17266
|
Probing the specificity of the subclass B3 FEZ-1 metallo-beta-lactamase by site-directed mutagenesis
|
|
17267
|
Probing the specificity of the subclass B3 FEZ-1 metallo-beta-lactamase by site-directed mutagenesis
|
|
17268
|
Probing the specificity of the subclass B3 FEZ-1 metallo-beta-lactamase by site-directed mutagenesis
|
|
17269
|
Probing the specificity of the subclass B3 FEZ-1 metallo-beta-lactamase by site-directed mutagenesis
|
|
17270
|
Probing the specificity of the subclass B3 FEZ-1 metallo-beta-lactamase by site-directed mutagenesis
|
|
17271
|
Probing the specificity of the subclass B3 FEZ-1 metallo-beta-lactamase by site-directed mutagenesis
|
|
17272
|
Probing the specificity of the subclass B3 FEZ-1 metallo-beta-lactamase by site-directed mutagenesis
|
|
17273
|
Probing the specificity of the subclass B3 FEZ-1 metallo-beta-lactamase by site-directed mutagenesis
|
|
17274
|
Probing the specificity of the subclass B3 FEZ-1 metallo-beta-lactamase by site-directed mutagenesis
|
|
17275
|
Probing the specificity of the subclass B3 FEZ-1 metallo-beta-lactamase by site-directed mutagenesis
|
|
17276
|
Probing the specificity of the subclass B3 FEZ-1 metallo-beta-lactamase by site-directed mutagenesis
|
|
17277
|
Probing the specificity of the subclass B3 FEZ-1 metallo-beta-lactamase by site-directed mutagenesis
|
|
17278
|
Probing the specificity of the subclass B3 FEZ-1 metallo-beta-lactamase by site-directed mutagenesis
|
|
17279
|
Probing the specificity of the subclass B3 FEZ-1 metallo-beta-lactamase by site-directed mutagenesis
|
|
17280
|
Probing the specificity of the subclass B3 FEZ-1 metallo-beta-lactamase by site-directed mutagenesis
|
|
17281
|
Probing the specificity of the subclass B3 FEZ-1 metallo-beta-lactamase by site-directed mutagenesis
|
|
17282
|
Probing the specificity of the subclass B3 FEZ-1 metallo-beta-lactamase by site-directed mutagenesis
|
|
17283
|
Probing the specificity of the subclass B3 FEZ-1 metallo-beta-lactamase by site-directed mutagenesis
|
|
17284
|
Probing the specificity of the subclass B3 FEZ-1 metallo-beta-lactamase by site-directed mutagenesis
|
|
17285
|
Probing the specificity of the subclass B3 FEZ-1 metallo-beta-lactamase by site-directed mutagenesis
|
|
17286
|
Probing the specificity of the subclass B3 FEZ-1 metallo-beta-lactamase by site-directed mutagenesis
|
|
17287
|
Probing the specificity of the subclass B3 FEZ-1 metallo-beta-lactamase by site-directed mutagenesis
|
|
17288
|
Probing the specificity of the subclass B3 FEZ-1 metallo-beta-lactamase by site-directed mutagenesis
|
|
17289
|
Probing the specificity of the subclass B3 FEZ-1 metallo-beta-lactamase by site-directed mutagenesis
|
|
17290
|
Probing the specificity of the subclass B3 FEZ-1 metallo-beta-lactamase by site-directed mutagenesis
|
|
17291
|
Probing the specificity of the subclass B3 FEZ-1 metallo-beta-lactamase by site-directed mutagenesis
|
|
17292
|
Probing the specificity of the subclass B3 FEZ-1 metallo-beta-lactamase by site-directed mutagenesis
|
|
17293
|
Probing the role of Asp-120(81) of metallo-beta-lactamase (IMP-1) by site-directed mutagenesis, kinetic ...
|
|
17294
|
Probing the role of Asp-120(81) of metallo-beta-lactamase (IMP-1) by site-directed mutagenesis, kinetic ...
|
|
17295
|
Probing the role of Asp-120(81) of metallo-beta-lactamase (IMP-1) by site-directed mutagenesis, kinetic ...
|
|
17296
|
Probing the role of Asp-120(81) of metallo-beta-lactamase (IMP-1) by site-directed mutagenesis, kinetic ...
|
|
17297
|
Probing the role of Asp-120(81) of metallo-beta-lactamase (IMP-1) by site-directed mutagenesis, kinetic ...
|
|
17298
|
Probing the role of Asp-120(81) of metallo-beta-lactamase (IMP-1) by site-directed mutagenesis, kinetic ...
|
|
17299
|
Probing the role of Asp-120(81) of metallo-beta-lactamase (IMP-1) by site-directed mutagenesis, kinetic ...
|
|
17300
|
Probing the role of Asp-120(81) of metallo-beta-lactamase (IMP-1) by site-directed mutagenesis, kinetic ...
|
|
17301
|
Probing the role of Asp-120(81) of metallo-beta-lactamase (IMP-1) by site-directed mutagenesis, kinetic ...
|
|
17302
|
Probing the role of Asp-120(81) of metallo-beta-lactamase (IMP-1) by site-directed mutagenesis, kinetic ...
|
|
17303
|
Probing the role of Asp-120(81) of metallo-beta-lactamase (IMP-1) by site-directed mutagenesis, kinetic ...
|
|
17304
|
Probing the role of Asp-120(81) of metallo-beta-lactamase (IMP-1) by site-directed mutagenesis, kinetic ...
|
|
17305
|
Probing the role of Asp-120(81) of metallo-beta-lactamase (IMP-1) by site-directed mutagenesis, kinetic ...
|
|
17306
|
Probing the role of Asp-120(81) of metallo-beta-lactamase (IMP-1) by site-directed mutagenesis, kinetic ...
|
|
17307
|
Probing the role of Asp-120(81) of metallo-beta-lactamase (IMP-1) by site-directed mutagenesis, kinetic ...
|
|
17308
|
Probing the role of Asp-120(81) of metallo-beta-lactamase (IMP-1) by site-directed mutagenesis, kinetic ...
|
|
17309
|
Probing the role of Asp-120(81) of metallo-beta-lactamase (IMP-1) by site-directed mutagenesis, kinetic ...
|
|
17310
|
Probing the role of Asp-120(81) of metallo-beta-lactamase (IMP-1) by site-directed mutagenesis, kinetic ...
|
|
17311
|
Probing the role of Asp-120(81) of metallo-beta-lactamase (IMP-1) by site-directed mutagenesis, kinetic ...
|
|
17312
|
Probing the role of Asp-120(81) of metallo-beta-lactamase (IMP-1) by site-directed mutagenesis, kinetic ...
|
|
17313
|
Probing the role of Asp-120(81) of metallo-beta-lactamase (IMP-1) by site-directed mutagenesis, kinetic ...
|
|
17314
|
Probing the role of Asp-120(81) of metallo-beta-lactamase (IMP-1) by site-directed mutagenesis, kinetic ...
|
|
17315
|
Probing the role of Asp-120(81) of metallo-beta-lactamase (IMP-1) by site-directed mutagenesis, kinetic ...
|
|
17316
|
Probing the role of Asp-120(81) of metallo-beta-lactamase (IMP-1) by site-directed mutagenesis, kinetic ...
|
|
17317
|
Probing the role of Asp-120(81) of metallo-beta-lactamase (IMP-1) by site-directed mutagenesis, kinetic ...
|
|
17318
|
Probing the role of Asp-120(81) of metallo-beta-lactamase (IMP-1) by site-directed mutagenesis, kinetic ...
|
|
17319
|
Probing the role of Asp-120(81) of metallo-beta-lactamase (IMP-1) by site-directed mutagenesis, kinetic ...
|
|
17320
|
Probing the role of Asp-120(81) of metallo-beta-lactamase (IMP-1) by site-directed mutagenesis, kinetic ...
|
|
17321
|
Probing the role of Asp-120(81) of metallo-beta-lactamase (IMP-1) by site-directed mutagenesis, kinetic ...
|
|
17322
|
Probing the role of Asp-120(81) of metallo-beta-lactamase (IMP-1) by site-directed mutagenesis, kinetic ...
|
|
17323
|
Probing the role of Asp-120(81) of metallo-beta-lactamase (IMP-1) by site-directed mutagenesis, kinetic ...
|
|
17324
|
Probing the role of Asp-120(81) of metallo-beta-lactamase (IMP-1) by site-directed mutagenesis, kinetic ...
|
|
17325
|
Probing the role of Asp-120(81) of metallo-beta-lactamase (IMP-1) by site-directed mutagenesis, kinetic ...
|
|
17326
|
Probing the role of Asp-120(81) of metallo-beta-lactamase (IMP-1) by site-directed mutagenesis, kinetic ...
|
|
17327
|
Probing the role of Asp-120(81) of metallo-beta-lactamase (IMP-1) by site-directed mutagenesis, kinetic ...
|
|
17328
|
Probing the role of Asp-120(81) of metallo-beta-lactamase (IMP-1) by site-directed mutagenesis, kinetic ...
|
|
17329
|
Caenorhabditis elegans has two genes encoding functional d-aspartate oxidases
|
|
17330
|
Caenorhabditis elegans has two genes encoding functional d-aspartate oxidases
|
|
17331
|
Caenorhabditis elegans has two genes encoding functional d-aspartate oxidases
|
|
17332
|
Caenorhabditis elegans has two genes encoding functional d-aspartate oxidases
|
|
17333
|
Caenorhabditis elegans has two genes encoding functional d-aspartate oxidases
|
|
17334
|
Caenorhabditis elegans has two genes encoding functional d-aspartate oxidases
|
|
17335
|
Caenorhabditis elegans has two genes encoding functional d-aspartate oxidases
|
|
17336
|
Caenorhabditis elegans has two genes encoding functional d-aspartate oxidases
|
|
17337
|
Caenorhabditis elegans has two genes encoding functional d-aspartate oxidases
|
|
17338
|
Alpha-keto aldehyde dehydrogenase, an enzyme that catalyzes the enzymic oxidation of methylglyoxal to pyruvate
|
|
17339
|
Alpha-keto aldehyde dehydrogenase, an enzyme that catalyzes the enzymic oxidation of methylglyoxal to pyruvate
|
|
17340
|
Alpha-keto aldehyde dehydrogenase, an enzyme that catalyzes the enzymic oxidation of methylglyoxal to pyruvate
|
|
17341
|
Alpha-keto aldehyde dehydrogenase, an enzyme that catalyzes the enzymic oxidation of methylglyoxal to pyruvate
|
|
17342
|
Alpha-keto aldehyde dehydrogenase, an enzyme that catalyzes the enzymic oxidation of methylglyoxal to pyruvate
|
|
17343
|
Alpha-keto aldehyde dehydrogenase, an enzyme that catalyzes the enzymic oxidation of methylglyoxal to pyruvate
|
|
17344
|
Alpha-keto aldehyde dehydrogenase, an enzyme that catalyzes the enzymic oxidation of methylglyoxal to pyruvate
|
|
17345
|
Alpha-keto aldehyde dehydrogenase, an enzyme that catalyzes the enzymic oxidation of methylglyoxal to pyruvate
|
|
17346
|
Alpha-keto aldehyde dehydrogenase, an enzyme that catalyzes the enzymic oxidation of methylglyoxal to pyruvate
|
|
17347
|
Alpha-keto aldehyde dehydrogenase, an enzyme that catalyzes the enzymic oxidation of methylglyoxal to pyruvate
|
|
17348
|
Alpha-keto aldehyde dehydrogenase, an enzyme that catalyzes the enzymic oxidation of methylglyoxal to pyruvate
|
|
17349
|
Alpha-keto aldehyde dehydrogenase, an enzyme that catalyzes the enzymic oxidation of methylglyoxal to pyruvate
|
|
17350
|
Alpha-keto aldehyde dehydrogenase, an enzyme that catalyzes the enzymic oxidation of methylglyoxal to pyruvate
|
|
17351
|
Alpha-keto aldehyde dehydrogenase, an enzyme that catalyzes the enzymic oxidation of methylglyoxal to pyruvate
|
|
17352
|
Alpha-keto aldehyde dehydrogenase, an enzyme that catalyzes the enzymic oxidation of methylglyoxal to pyruvate
|
|
17353
|
Alpha-keto aldehyde dehydrogenase, an enzyme that catalyzes the enzymic oxidation of methylglyoxal to pyruvate
|
|
17354
|
Isoenzymes of phosphofructokinase in the rat. Demonstration of the three non-identical subunits by ...
|
|
17355
|
Isoenzymes of phosphofructokinase in the rat. Demonstration of the three non-identical subunits by ...
|
|
17356
|
Isoenzymes of phosphofructokinase in the rat. Demonstration of the three non-identical subunits by ...
|
|
17357
|
Isoenzymes of phosphofructokinase in the rat. Demonstration of the three non-identical subunits by ...
|
|
17358
|
Isoenzymes of phosphofructokinase in the rat. Demonstration of the three non-identical subunits by ...
|
|
17359
|
Isoenzymes of phosphofructokinase in the rat. Demonstration of the three non-identical subunits by ...
|
|
17360
|
Isoenzymes of phosphofructokinase in the rat. Demonstration of the three non-identical subunits by ...
|
|
17361
|
Isoenzymes of phosphofructokinase in the rat. Demonstration of the three non-identical subunits by ...
|
|
17362
|
Isoenzymes of phosphofructokinase in the rat. Demonstration of the three non-identical subunits by ...
|
|
17363
|
New insights from the structure-function analysis of the catalytic region of human platelet phosphodiesterase ...
|
|
17364
|
New insights from the structure-function analysis of the catalytic region of human platelet phosphodiesterase ...
|
|
17365
|
New insights from the structure-function analysis of the catalytic region of human platelet phosphodiesterase ...
|
|
17366
|
New insights from the structure-function analysis of the catalytic region of human platelet phosphodiesterase ...
|
|
17367
|
New insights from the structure-function analysis of the catalytic region of human platelet phosphodiesterase ...
|
|
17368
|
New insights from the structure-function analysis of the catalytic region of human platelet phosphodiesterase ...
|
|
17369
|
New insights from the structure-function analysis of the catalytic region of human platelet phosphodiesterase ...
|
|
17370
|
New insights from the structure-function analysis of the catalytic region of human platelet phosphodiesterase ...
|
|
17371
|
New insights from the structure-function analysis of the catalytic region of human platelet phosphodiesterase ...
|
|
17372
|
New insights from the structure-function analysis of the catalytic region of human platelet phosphodiesterase ...
|
|
17373
|
New insights from the structure-function analysis of the catalytic region of human platelet phosphodiesterase ...
|
|
17374
|
New insights from the structure-function analysis of the catalytic region of human platelet phosphodiesterase ...
|
|
17375
|
New insights from the structure-function analysis of the catalytic region of human platelet phosphodiesterase ...
|
|
17376
|
New insights from the structure-function analysis of the catalytic region of human platelet phosphodiesterase ...
|
|
17377
|
Enzymes for the NADPH-dependent reduction of dihydroxyacetone and D-glyceraldehyde and L-glyceraldehyde in the ...
|
|
17378
|
Enzymes for the NADPH-dependent reduction of dihydroxyacetone and D-glyceraldehyde and L-glyceraldehyde in the ...
|
|
17379
|
Enzymes for the NADPH-dependent reduction of dihydroxyacetone and D-glyceraldehyde and L-glyceraldehyde in the ...
|
|
17380
|
Enzymes for the NADPH-dependent reduction of dihydroxyacetone and D-glyceraldehyde and L-glyceraldehyde in the ...
|
|
17381
|
Enzymes for the NADPH-dependent reduction of dihydroxyacetone and D-glyceraldehyde and L-glyceraldehyde in the ...
|
|
17382
|
Enzymes for the NADPH-dependent reduction of dihydroxyacetone and D-glyceraldehyde and L-glyceraldehyde in the ...
|
|
17383
|
Enzymes for the NADPH-dependent reduction of dihydroxyacetone and D-glyceraldehyde and L-glyceraldehyde in the ...
|
|
17384
|
Enzymes for the NADPH-dependent reduction of dihydroxyacetone and D-glyceraldehyde and L-glyceraldehyde in the ...
|
|
17385
|
Enzymes for the NADPH-dependent reduction of dihydroxyacetone and D-glyceraldehyde and L-glyceraldehyde in the ...
|
|
17386
|
Enzymes for the NADPH-dependent reduction of dihydroxyacetone and D-glyceraldehyde and L-glyceraldehyde in the ...
|
|
17387
|
Enzymes for the NADPH-dependent reduction of dihydroxyacetone and D-glyceraldehyde and L-glyceraldehyde in the ...
|
|
17388
|
Enzymes for the NADPH-dependent reduction of dihydroxyacetone and D-glyceraldehyde and L-glyceraldehyde in the ...
|
|
17389
|
Enzymes for the NADPH-dependent reduction of dihydroxyacetone and D-glyceraldehyde and L-glyceraldehyde in the ...
|
|
17390
|
Enzymes for the NADPH-dependent reduction of dihydroxyacetone and D-glyceraldehyde and L-glyceraldehyde in the ...
|
|
17391
|
Enzymes for the NADPH-dependent reduction of dihydroxyacetone and D-glyceraldehyde and L-glyceraldehyde in the ...
|
|
17392
|
Enzymes for the NADPH-dependent reduction of dihydroxyacetone and D-glyceraldehyde and L-glyceraldehyde in the ...
|
|
17393
|
Enzymes for the NADPH-dependent reduction of dihydroxyacetone and D-glyceraldehyde and L-glyceraldehyde in the ...
|
|
17394
|
Enzymes for the NADPH-dependent reduction of dihydroxyacetone and D-glyceraldehyde and L-glyceraldehyde in the ...
|
|
17395
|
Purification and characterization of guinea pig liver morphine 6-dehydrogenase
|
|
17396
|
Purification and characterization of guinea pig liver morphine 6-dehydrogenase
|
|
17397
|
Purification and characterization of guinea pig liver morphine 6-dehydrogenase
|
|
17398
|
Purification and characterization of guinea pig liver morphine 6-dehydrogenase
|
|
17399
|
Purification and characterization of guinea pig liver morphine 6-dehydrogenase
|
|
17400
|
Purification and characterization of guinea pig liver morphine 6-dehydrogenase
|
|
17401
|
Purification and characterization of guinea pig liver morphine 6-dehydrogenase
|
|
17402
|
Purification and characterization of guinea pig liver morphine 6-dehydrogenase
|
|
17403
|
Purification and characterization of guinea pig liver morphine 6-dehydrogenase
|
|
17404
|
Purification and characterization of guinea pig liver morphine 6-dehydrogenase
|
|
17405
|
Purification and characterization of guinea pig liver morphine 6-dehydrogenase
|
|
17406
|
Purification and characterization of guinea pig liver morphine 6-dehydrogenase
|
|
17407
|
Purification and characterization of guinea pig liver morphine 6-dehydrogenase
|
|
17408
|
Purification and characterization of guinea pig liver morphine 6-dehydrogenase
|
|
17409
|
Purification and characterization of guinea pig liver morphine 6-dehydrogenase
|
|
17410
|
Purification and characterization of guinea pig liver morphine 6-dehydrogenase
|
|
17411
|
Purification and characterization of guinea pig liver morphine 6-dehydrogenase
|
|
17412
|
Purification and characterization of guinea pig liver morphine 6-dehydrogenase
|
|
17413
|
Purification and characterization of guinea pig liver morphine 6-dehydrogenase
|
|
17414
|
Purification and characterization of guinea pig liver morphine 6-dehydrogenase
|
|
17415
|
Modification of activity and specificity of haloalkane dehalogenase from Sphingomonas paucimobilis UT26 by ...
|
|
17416
|
Modification of activity and specificity of haloalkane dehalogenase from Sphingomonas paucimobilis UT26 by ...
|
|
17417
|
Modification of activity and specificity of haloalkane dehalogenase from Sphingomonas paucimobilis UT26 by ...
|
|
17418
|
Modification of activity and specificity of haloalkane dehalogenase from Sphingomonas paucimobilis UT26 by ...
|
|
17419
|
Modification of activity and specificity of haloalkane dehalogenase from Sphingomonas paucimobilis UT26 by ...
|
|
17420
|
Modification of activity and specificity of haloalkane dehalogenase from Sphingomonas paucimobilis UT26 by ...
|
|
17421
|
Modification of activity and specificity of haloalkane dehalogenase from Sphingomonas paucimobilis UT26 by ...
|
|
17422
|
Modification of activity and specificity of haloalkane dehalogenase from Sphingomonas paucimobilis UT26 by ...
|
|
17423
|
Modification of activity and specificity of haloalkane dehalogenase from Sphingomonas paucimobilis UT26 by ...
|
|
17424
|
Modification of activity and specificity of haloalkane dehalogenase from Sphingomonas paucimobilis UT26 by ...
|
|
17425
|
Modification of activity and specificity of haloalkane dehalogenase from Sphingomonas paucimobilis UT26 by ...
|
|
17426
|
Modification of activity and specificity of haloalkane dehalogenase from Sphingomonas paucimobilis UT26 by ...
|
|
17427
|
Modification of activity and specificity of haloalkane dehalogenase from Sphingomonas paucimobilis UT26 by ...
|
|
17428
|
Modification of activity and specificity of haloalkane dehalogenase from Sphingomonas paucimobilis UT26 by ...
|
|
17429
|
Modification of activity and specificity of haloalkane dehalogenase from Sphingomonas paucimobilis UT26 by ...
|
|
17430
|
Modification of activity and specificity of haloalkane dehalogenase from Sphingomonas paucimobilis UT26 by ...
|
|
17431
|
Modification of activity and specificity of haloalkane dehalogenase from Sphingomonas paucimobilis UT26 by ...
|
|
17432
|
Modification of activity and specificity of haloalkane dehalogenase from Sphingomonas paucimobilis UT26 by ...
|
|
17433
|
Modification of activity and specificity of haloalkane dehalogenase from Sphingomonas paucimobilis UT26 by ...
|
|
17434
|
Modification of activity and specificity of haloalkane dehalogenase from Sphingomonas paucimobilis UT26 by ...
|
|
17435
|
Modification of activity and specificity of haloalkane dehalogenase from Sphingomonas paucimobilis UT26 by ...
|
|
17436
|
Modification of activity and specificity of haloalkane dehalogenase from Sphingomonas paucimobilis UT26 by ...
|
|
17437
|
Modification of activity and specificity of haloalkane dehalogenase from Sphingomonas paucimobilis UT26 by ...
|
|
17438
|
Modification of activity and specificity of haloalkane dehalogenase from Sphingomonas paucimobilis UT26 by ...
|
|
17439
|
Modification of activity and specificity of haloalkane dehalogenase from Sphingomonas paucimobilis UT26 by ...
|
|
17440
|
Modification of activity and specificity of haloalkane dehalogenase from Sphingomonas paucimobilis UT26 by ...
|
|
17441
|
Modification of activity and specificity of haloalkane dehalogenase from Sphingomonas paucimobilis UT26 by ...
|
|
17442
|
Modification of activity and specificity of haloalkane dehalogenase from Sphingomonas paucimobilis UT26 by ...
|
|
17443
|
Modification of activity and specificity of haloalkane dehalogenase from Sphingomonas paucimobilis UT26 by ...
|
|
17444
|
Modification of activity and specificity of haloalkane dehalogenase from Sphingomonas paucimobilis UT26 by ...
|
|
17445
|
Modification of activity and specificity of haloalkane dehalogenase from Sphingomonas paucimobilis UT26 by ...
|
|
17446
|
Modification of activity and specificity of haloalkane dehalogenase from Sphingomonas paucimobilis UT26 by ...
|
|
17447
|
Modification of activity and specificity of haloalkane dehalogenase from Sphingomonas paucimobilis UT26 by ...
|
|
17448
|
Effects of flavin-binding motif amino acid mutations in the NADH-cytochrome b5 reductase catalytic domain on ...
|
|
17449
|
Effects of flavin-binding motif amino acid mutations in the NADH-cytochrome b5 reductase catalytic domain on ...
|
|
17450
|
Effects of flavin-binding motif amino acid mutations in the NADH-cytochrome b5 reductase catalytic domain on ...
|
|
17451
|
Effects of flavin-binding motif amino acid mutations in the NADH-cytochrome b5 reductase catalytic domain on ...
|
|
17452
|
Effects of flavin-binding motif amino acid mutations in the NADH-cytochrome b5 reductase catalytic domain on ...
|
|
17453
|
Effects of flavin-binding motif amino acid mutations in the NADH-cytochrome b5 reductase catalytic domain on ...
|
|
17454
|
Effects of flavin-binding motif amino acid mutations in the NADH-cytochrome b5 reductase catalytic domain on ...
|
|
17455
|
Effects of flavin-binding motif amino acid mutations in the NADH-cytochrome b5 reductase catalytic domain on ...
|
|
17456
|
Effects of flavin-binding motif amino acid mutations in the NADH-cytochrome b5 reductase catalytic domain on ...
|
|
17457
|
Effects of flavin-binding motif amino acid mutations in the NADH-cytochrome b5 reductase catalytic domain on ...
|
|
17458
|
Effects of flavin-binding motif amino acid mutations in the NADH-cytochrome b5 reductase catalytic domain on ...
|
|
17459
|
Effects of flavin-binding motif amino acid mutations in the NADH-cytochrome b5 reductase catalytic domain on ...
|
|
17460
|
Effects of flavin-binding motif amino acid mutations in the NADH-cytochrome b5 reductase catalytic domain on ...
|
|
17461
|
Effects of flavin-binding motif amino acid mutations in the NADH-cytochrome b5 reductase catalytic domain on ...
|
|
17462
|
Effects of flavin-binding motif amino acid mutations in the NADH-cytochrome b5 reductase catalytic domain on ...
|
|
17463
|
Effects of flavin-binding motif amino acid mutations in the NADH-cytochrome b5 reductase catalytic domain on ...
|
|
17464
|
Effects of flavin-binding motif amino acid mutations in the NADH-cytochrome b5 reductase catalytic domain on ...
|
|
17465
|
Effects of flavin-binding motif amino acid mutations in the NADH-cytochrome b5 reductase catalytic domain on ...
|
|
17466
|
Effects of flavin-binding motif amino acid mutations in the NADH-cytochrome b5 reductase catalytic domain on ...
|
|
17467
|
Effects of flavin-binding motif amino acid mutations in the NADH-cytochrome b5 reductase catalytic domain on ...
|
|
17468
|
Effects of flavin-binding motif amino acid mutations in the NADH-cytochrome b5 reductase catalytic domain on ...
|
|
17469
|
Effects of flavin-binding motif amino acid mutations in the NADH-cytochrome b5 reductase catalytic domain on ...
|
|
17470
|
Mg2+ affects the binding of ADP but not ATP to 3-phosphoglycerate kinase. Correlation between equilibrium ...
|
|
17471
|
Mg2+ affects the binding of ADP but not ATP to 3-phosphoglycerate kinase. Correlation between equilibrium ...
|
|
17472
|
Mg2+ affects the binding of ADP but not ATP to 3-phosphoglycerate kinase. Correlation between equilibrium ...
|
|
17473
|
Mg2+ affects the binding of ADP but not ATP to 3-phosphoglycerate kinase. Correlation between equilibrium ...
|
|
17474
|
Mg2+ affects the binding of ADP but not ATP to 3-phosphoglycerate kinase. Correlation between equilibrium ...
|
|
17475
|
Mg2+ affects the binding of ADP but not ATP to 3-phosphoglycerate kinase. Correlation between equilibrium ...
|
|
17476
|
Mg2+ affects the binding of ADP but not ATP to 3-phosphoglycerate kinase. Correlation between equilibrium ...
|
|
17477
|
Mg2+ affects the binding of ADP but not ATP to 3-phosphoglycerate kinase. Correlation between equilibrium ...
|
|
17478
|
Mg2+ affects the binding of ADP but not ATP to 3-phosphoglycerate kinase. Correlation between equilibrium ...
|
|
17479
|
Mg2+ affects the binding of ADP but not ATP to 3-phosphoglycerate kinase. Correlation between equilibrium ...
|
|
17480
|
Mg2+ affects the binding of ADP but not ATP to 3-phosphoglycerate kinase. Correlation between equilibrium ...
|
|
17481
|
Mg2+ affects the binding of ADP but not ATP to 3-phosphoglycerate kinase. Correlation between equilibrium ...
|
|
17482
|
Mg2+ affects the binding of ADP but not ATP to 3-phosphoglycerate kinase. Correlation between equilibrium ...
|
|
17483
|
Amine N-methyltransferases from rabbit liver
|
|
17484
|
Amine N-methyltransferases from rabbit liver
|
|
17485
|
Amine N-methyltransferases from rabbit liver
|
|
17486
|
Amine N-methyltransferases from rabbit liver
|
|
17487
|
Amine N-methyltransferases from rabbit liver
|
|
17488
|
Amine N-methyltransferases from rabbit liver
|
|
17489
|
Amine N-methyltransferases from rabbit liver
|
|
17490
|
Amine N-methyltransferases from rabbit liver
|
|
17491
|
Amine N-methyltransferases from rabbit liver
|
|
17492
|
Amine N-methyltransferases from rabbit liver
|
|
17493
|
Amine N-methyltransferases from rabbit liver
|
|
17494
|
Amine N-methyltransferases from rabbit liver
|
|
17495
|
Amine N-methyltransferases from rabbit liver
|
|
17496
|
Amine N-methyltransferases from rabbit liver
|
|
17497
|
Amine N-methyltransferases from rabbit liver
|
|
17498
|
Amine N-methyltransferases from rabbit liver
|
|
17499
|
Identification and characterization of a new mammalian glutaredoxin (thioltransferase), Grx2
|
|
17500
|
Identification and characterization of a new mammalian glutaredoxin (thioltransferase), Grx2
|
|
17501
|
Identification and characterization of a new mammalian glutaredoxin (thioltransferase), Grx2
|
|
17502
|
Further studies of the role of Ser-16 in the regulation of the activity of phenylalanine hydroxylase
|
|
17503
|
Further studies of the role of Ser-16 in the regulation of the activity of phenylalanine hydroxylase
|
|
17504
|
Further studies of the role of Ser-16 in the regulation of the activity of phenylalanine hydroxylase
|
|
17505
|
Further studies of the role of Ser-16 in the regulation of the activity of phenylalanine hydroxylase
|
|
17506
|
Further studies of the role of Ser-16 in the regulation of the activity of phenylalanine hydroxylase
|
|
17507
|
Further studies of the role of Ser-16 in the regulation of the activity of phenylalanine hydroxylase
|
|
17508
|
Further studies of the role of Ser-16 in the regulation of the activity of phenylalanine hydroxylase
|
|
17509
|
Further studies of the role of Ser-16 in the regulation of the activity of phenylalanine hydroxylase
|
|
17510
|
Further studies of the role of Ser-16 in the regulation of the activity of phenylalanine hydroxylase
|
|
17511
|
Further studies of the role of Ser-16 in the regulation of the activity of phenylalanine hydroxylase
|
|
17512
|
Further studies of the role of Ser-16 in the regulation of the activity of phenylalanine hydroxylase
|
|
17513
|
Further studies of the role of Ser-16 in the regulation of the activity of phenylalanine hydroxylase
|
|
17514
|
Further studies of the role of Ser-16 in the regulation of the activity of phenylalanine hydroxylase
|
|
17515
|
Further studies of the role of Ser-16 in the regulation of the activity of phenylalanine hydroxylase
|
|
17516
|
Further studies of the role of Ser-16 in the regulation of the activity of phenylalanine hydroxylase
|
|
17517
|
Further studies of the role of Ser-16 in the regulation of the activity of phenylalanine hydroxylase
|
|
17518
|
Purification and properties of the aromatic amino acid aminotransferase from gramicidin S-producing Bacillus ...
|
|
17519
|
Purification and properties of the aromatic amino acid aminotransferase from gramicidin S-producing Bacillus ...
|
|
17520
|
Purification and properties of the aromatic amino acid aminotransferase from gramicidin S-producing Bacillus ...
|
|
17521
|
Purification and properties of the aromatic amino acid aminotransferase from gramicidin S-producing Bacillus ...
|
|
17522
|
Purification and properties of the aromatic amino acid aminotransferase from gramicidin S-producing Bacillus ...
|
|
17523
|
Purification and properties of the aromatic amino acid aminotransferase from gramicidin S-producing Bacillus ...
|
|
17524
|
Purification and properties of the aromatic amino acid aminotransferase from gramicidin S-producing Bacillus ...
|
|
17525
|
Purification and properties of the aromatic amino acid aminotransferase from gramicidin S-producing Bacillus ...
|
|
17526
|
Purification and properties of the aromatic amino acid aminotransferase from gramicidin S-producing Bacillus ...
|
|
17527
|
Purification and properties of the aromatic amino acid aminotransferase from gramicidin S-producing Bacillus ...
|
|
17528
|
Purification and properties of the aromatic amino acid aminotransferase from gramicidin S-producing Bacillus ...
|
|
17529
|
Purification and properties of the aromatic amino acid aminotransferase from gramicidin S-producing Bacillus ...
|
|
17530
|
Purification and properties of the aromatic amino acid aminotransferase from gramicidin S-producing Bacillus ...
|
|
17531
|
Purification and properties of the aromatic amino acid aminotransferase from gramicidin S-producing Bacillus ...
|
|
17532
|
Purification and properties of the aromatic amino acid aminotransferase from gramicidin S-producing Bacillus ...
|
|
17533
|
Purification and properties of the aromatic amino acid aminotransferase from gramicidin S-producing Bacillus ...
|
|
17534
|
Purification and properties of the aromatic amino acid aminotransferase from gramicidin S-producing Bacillus ...
|
|
17535
|
Purification and properties of the aromatic amino acid aminotransferase from gramicidin S-producing Bacillus ...
|
|
17536
|
Purification and characterization of cytosol phosphoenolpyruvate carboxykinase from bullfrog (Rana ...
|
|
17537
|
Purification and characterization of cytosol phosphoenolpyruvate carboxykinase from bullfrog (Rana ...
|
|
17538
|
Purification and characterization of cytosol phosphoenolpyruvate carboxykinase from bullfrog (Rana ...
|
|
17539
|
Purification and characterization of cytosol phosphoenolpyruvate carboxykinase from bullfrog (Rana ...
|
|
17540
|
Purification and characterization of cytosol phosphoenolpyruvate carboxykinase from bullfrog (Rana ...
|
|
17541
|
Purification and characterization of cytosol phosphoenolpyruvate carboxykinase from bullfrog (Rana ...
|
|
17542
|
Purification and characterization of cytosol phosphoenolpyruvate carboxykinase from bullfrog (Rana ...
|
|
17543
|
Purification and characterization of cytosol phosphoenolpyruvate carboxykinase from bullfrog (Rana ...
|
|
17544
|
Purification and characterization of cytosol phosphoenolpyruvate carboxykinase from bullfrog (Rana ...
|
|
17545
|
Purification and characterization of cytosol phosphoenolpyruvate carboxykinase from bullfrog (Rana ...
|
|
17546
|
Purification and characterization of cytosol phosphoenolpyruvate carboxykinase from bullfrog (Rana ...
|
|
17547
|
Purification and characterization of cytosol phosphoenolpyruvate carboxykinase from bullfrog (Rana ...
|
|
17548
|
Purification and characterization of cytosol phosphoenolpyruvate carboxykinase from bullfrog (Rana ...
|
|
17580
|
Kinetic properties and regulation by L-ornithine of chicken liver arginase induced by insulin
|
|
17581
|
Kinetic properties and regulation by L-ornithine of chicken liver arginase induced by insulin
|
|
17582
|
Kinetic properties and regulation by L-ornithine of chicken liver arginase induced by insulin
|
|
17589
|
Isolation and characterization of multiforms of aldehyde reductase in chicken kidney
|
|
17590
|
Isolation and characterization of multiforms of aldehyde reductase in chicken kidney
|
|
17591
|
Isolation and characterization of multiforms of aldehyde reductase in chicken kidney
|
|
17592
|
Isolation and characterization of multiforms of aldehyde reductase in chicken kidney
|
|
17593
|
Isolation and characterization of multiforms of aldehyde reductase in chicken kidney
|
|
17594
|
Isolation and characterization of multiforms of aldehyde reductase in chicken kidney
|
|
17595
|
Isolation and characterization of multiforms of aldehyde reductase in chicken kidney
|
|
17596
|
Isolation and characterization of multiforms of aldehyde reductase in chicken kidney
|
|
17597
|
Isolation and characterization of multiforms of aldehyde reductase in chicken kidney
|
|
17598
|
Isolation and characterization of multiforms of aldehyde reductase in chicken kidney
|
|
17599
|
Isolation and characterization of multiforms of aldehyde reductase in chicken kidney
|
|
17600
|
Isolation and characterization of multiforms of aldehyde reductase in chicken kidney
|
|
17601
|
Isolation and characterization of multiforms of aldehyde reductase in chicken kidney
|
|
17602
|
Isolation and characterization of multiforms of aldehyde reductase in chicken kidney
|
|
17603
|
Isolation and characterization of multiforms of aldehyde reductase in chicken kidney
|
|
17604
|
Isolation and characterization of multiforms of aldehyde reductase in chicken kidney
|
|
17605
|
Isolation and characterization of multiforms of aldehyde reductase in chicken kidney
|
|
17606
|
Isolation and characterization of multiforms of aldehyde reductase in chicken kidney
|
|
17607
|
Isolation and characterization of multiforms of aldehyde reductase in chicken kidney
|
|
17608
|
Isolation and characterization of multiforms of aldehyde reductase in chicken kidney
|
|
17609
|
Isolation and characterization of multiforms of aldehyde reductase in chicken kidney
|
|
17610
|
Isolation and characterization of multiforms of aldehyde reductase in chicken kidney
|
|
17611
|
Isolation and characterization of multiforms of aldehyde reductase in chicken kidney
|
|
17612
|
Isolation and characterization of multiforms of aldehyde reductase in chicken kidney
|
|
17613
|
Isolation and characterization of multiforms of aldehyde reductase in chicken kidney
|
|
17614
|
Isolation and characterization of multiforms of aldehyde reductase in chicken kidney
|
|
17615
|
Isolation and characterization of multiforms of aldehyde reductase in chicken kidney
|
|
17616
|
Isolation and characterization of multiforms of aldehyde reductase in chicken kidney
|
|
17617
|
Isolation and characterization of multiforms of aldehyde reductase in chicken kidney
|
|
17618
|
Isolation and characterization of multiforms of aldehyde reductase in chicken kidney
|
|
17619
|
Isolation and characterization of multiforms of aldehyde reductase in chicken kidney
|
|
17620
|
Isolation and characterization of multiforms of aldehyde reductase in chicken kidney
|
|
17621
|
Isolation and characterization of multiforms of aldehyde reductase in chicken kidney
|
|
17622
|
Isolation and characterization of multiforms of aldehyde reductase in chicken kidney
|
|
17623
|
Isolation and characterization of multiforms of aldehyde reductase in chicken kidney
|
|
17624
|
Isolation and characterization of multiforms of aldehyde reductase in chicken kidney
|
|
17625
|
Isolation and characterization of multiforms of aldehyde reductase in chicken kidney
|
|
17626
|
Isolation and characterization of multiforms of aldehyde reductase in chicken kidney
|
|
17627
|
Isolation and characterization of multiforms of aldehyde reductase in chicken kidney
|
|
17628
|
Isolation and characterization of multiforms of aldehyde reductase in chicken kidney
|
|
17629
|
Isolation and characterization of multiforms of aldehyde reductase in chicken kidney
|
|
17630
|
Isolation and characterization of multiforms of aldehyde reductase in chicken kidney
|
|
17631
|
Isolation and characterization of multiforms of aldehyde reductase in chicken kidney
|
|
17632
|
Isolation and characterization of multiforms of aldehyde reductase in chicken kidney
|
|
17633
|
Isolation and characterization of multiforms of aldehyde reductase in chicken kidney
|
|
17634
|
Isolation and characterization of multiforms of aldehyde reductase in chicken kidney
|
|
17635
|
Isolation and characterization of multiforms of aldehyde reductase in chicken kidney
|
|
17636
|
Isolation and characterization of multiforms of aldehyde reductase in chicken kidney
|
|
17637
|
Isolation and characterization of multiforms of aldehyde reductase in chicken kidney
|
|
17638
|
Isolation and characterization of multiforms of aldehyde reductase in chicken kidney
|
|
17639
|
Isolation and characterization of multiforms of aldehyde reductase in chicken kidney
|
|
17640
|
Isolation and characterization of multiforms of aldehyde reductase in chicken kidney
|
|
17641
|
Isolation and characterization of multiforms of aldehyde reductase in chicken kidney
|
|
17642
|
Isolation and characterization of multiforms of aldehyde reductase in chicken kidney
|
|
17643
|
Isolation and characterization of multiforms of aldehyde reductase in chicken kidney
|
|
17644
|
Isolation and characterization of multiforms of aldehyde reductase in chicken kidney
|
|
17645
|
Isolation and characterization of multiforms of aldehyde reductase in chicken kidney
|
|
17646
|
Isolation and characterization of multiforms of aldehyde reductase in chicken kidney
|
|
17647
|
Isolation and characterization of multiforms of aldehyde reductase in chicken kidney
|
|
17648
|
Isolation and characterization of multiforms of aldehyde reductase in chicken kidney
|
|
17649
|
Isolation and characterization of multiforms of aldehyde reductase in chicken kidney
|
|
17650
|
Isolation and characterization of multiforms of aldehyde reductase in chicken kidney
|
|
17651
|
Isolation and characterization of multiforms of aldehyde reductase in chicken kidney
|
|
17652
|
Isolation and characterization of multiforms of aldehyde reductase in chicken kidney
|
|
17653
|
Isolation and characterization of multiforms of aldehyde reductase in chicken kidney
|
|
17654
|
Isolation and characterization of multiforms of aldehyde reductase in chicken kidney
|
|
17655
|
Isolation and characterization of multiforms of aldehyde reductase in chicken kidney
|
|
17656
|
Isolation and characterization of multiforms of aldehyde reductase in chicken kidney
|
|
17657
|
Isolation and characterization of multiforms of aldehyde reductase in chicken kidney
|
|
17658
|
Isolation and characterization of multiforms of aldehyde reductase in chicken kidney
|
|
17659
|
Isolation and characterization of multiforms of aldehyde reductase in chicken kidney
|
|
17660
|
Isolation and characterization of multiforms of aldehyde reductase in chicken kidney
|
|
17661
|
Isolation and characterization of multiforms of aldehyde reductase in chicken kidney
|
|
17662
|
Isolation and characterization of multiforms of aldehyde reductase in chicken kidney
|
|
17663
|
Isolation and characterization of multiforms of aldehyde reductase in chicken kidney
|
|
17664
|
Isolation and characterization of multiforms of aldehyde reductase in chicken kidney
|
|
17665
|
Isolation and characterization of multiforms of aldehyde reductase in chicken kidney
|
|
17666
|
Isolation and characterization of multiforms of aldehyde reductase in chicken kidney
|
|
17667
|
Isolation and characterization of multiforms of aldehyde reductase in chicken kidney
|
|
17668
|
Isolation and characterization of multiforms of aldehyde reductase in chicken kidney
|
|
17669
|
Isolation and characterization of multiforms of aldehyde reductase in chicken kidney
|
|
17670
|
Isolation and characterization of multiforms of aldehyde reductase in chicken kidney
|
|
17671
|
Isolation and characterization of multiforms of aldehyde reductase in chicken kidney
|
|
17672
|
Isolation and characterization of multiforms of aldehyde reductase in chicken kidney
|
|
17673
|
Isolation and characterization of multiforms of aldehyde reductase in chicken kidney
|
|
17674
|
Isolation and characterization of multiforms of aldehyde reductase in chicken kidney
|
|
17675
|
Isolation and characterization of multiforms of aldehyde reductase in chicken kidney
|
|
17676
|
Isolation and characterization of multiforms of aldehyde reductase in chicken kidney
|
|
17677
|
Isolation and characterization of multiforms of aldehyde reductase in chicken kidney
|
|
17678
|
Isolation and characterization of multiforms of aldehyde reductase in chicken kidney
|
|
17679
|
Regulation of lactose catabolism in Streptococcus mutans: purification and regulatory properties of ...
|
|
17680
|
Regulation of lactose catabolism in Streptococcus mutans: purification and regulatory properties of ...
|
|
17681
|
Regulation of lactose catabolism in Streptococcus mutans: purification and regulatory properties of ...
|
|
17682
|
Regulation of lactose catabolism in Streptococcus mutans: purification and regulatory properties of ...
|
|
17683
|
Regulation of lactose catabolism in Streptococcus mutans: purification and regulatory properties of ...
|
|
17684
|
Characterization of rat and human UDP-glucuronosyltransferases responsible for the in vitro glucuronidation of ...
|
|
17685
|
Characterization of rat and human UDP-glucuronosyltransferases responsible for the in vitro glucuronidation of ...
|
|
17686
|
Characterization of rat and human UDP-glucuronosyltransferases responsible for the in vitro glucuronidation of ...
|
|
17687
|
Characterization of rat and human UDP-glucuronosyltransferases responsible for the in vitro glucuronidation of ...
|
|
17688
|
Characterization of rat and human UDP-glucuronosyltransferases responsible for the in vitro glucuronidation of ...
|
|
17689
|
Purine Nucleoside Phosphorylase. 1. Structure-Function Studies
|
|
17690
|
Purine Nucleoside Phosphorylase. 1. Structure-Function Studies
|
|
17691
|
Purine Nucleoside Phosphorylase. 1. Structure-Function Studies
|
|
17692
|
Purine Nucleoside Phosphorylase. 1. Structure-Function Studies
|
|
17693
|
Purine Nucleoside Phosphorylase. 1. Structure-Function Studies
|
|
17694
|
Purine Nucleoside Phosphorylase. 1. Structure-Function Studies
|
|
17695
|
Purine Nucleoside Phosphorylase. 1. Structure-Function Studies
|
|
17696
|
Purine Nucleoside Phosphorylase. 1. Structure-Function Studies
|
|
17697
|
Purine Nucleoside Phosphorylase. 1. Structure-Function Studies
|
|
17698
|
Purine Nucleoside Phosphorylase. 1. Structure-Function Studies
|
|
17699
|
Purine Nucleoside Phosphorylase. 1. Structure-Function Studies
|
|
17700
|
Purine Nucleoside Phosphorylase. 1. Structure-Function Studies
|
|
17701
|
Purine Nucleoside Phosphorylase. 1. Structure-Function Studies
|
|
17702
|
Purine Nucleoside Phosphorylase. 1. Structure-Function Studies
|
|
17703
|
Purine Nucleoside Phosphorylase. 1. Structure-Function Studies
|
|
17704
|
Purine Nucleoside Phosphorylase. 1. Structure-Function Studies
|
|
17705
|
Purine Nucleoside Phosphorylase. 1. Structure-Function Studies
|
|
17706
|
Purine Nucleoside Phosphorylase. 1. Structure-Function Studies
|
|
17707
|
Purine Nucleoside Phosphorylase. 1. Structure-Function Studies
|
|
17708
|
Purine Nucleoside Phosphorylase. 1. Structure-Function Studies
|
|
17709
|
Purine Nucleoside Phosphorylase. 1. Structure-Function Studies
|
|
17710
|
Purine Nucleoside Phosphorylase. 1. Structure-Function Studies
|
|
17711
|
Purine Nucleoside Phosphorylase. 1. Structure-Function Studies
|
|
17712
|
Purine Nucleoside Phosphorylase. 1. Structure-Function Studies
|
|
17713
|
Purine Nucleoside Phosphorylase. 1. Structure-Function Studies
|
|
17714
|
Purine Nucleoside Phosphorylase. 1. Structure-Function Studies
|
|
17715
|
Purine Nucleoside Phosphorylase. 1. Structure-Function Studies
|
|
17716
|
Purine Nucleoside Phosphorylase. 1. Structure-Function Studies
|
|
17717
|
Purine Nucleoside Phosphorylase. 1. Structure-Function Studies
|
|
17718
|
Purine Nucleoside Phosphorylase. 1. Structure-Function Studies
|
|
17719
|
Purine Nucleoside Phosphorylase. 1. Structure-Function Studies
|
|
17720
|
Purine Nucleoside Phosphorylase. 1. Structure-Function Studies
|
|
17721
|
Purine Nucleoside Phosphorylase. 1. Structure-Function Studies
|
|
17722
|
Purine Nucleoside Phosphorylase. 1. Structure-Function Studies
|
|
17723
|
Purine Nucleoside Phosphorylase. 1. Structure-Function Studies
|
|
17724
|
Purine Nucleoside Phosphorylase. 1. Structure-Function Studies
|
|
17725
|
Purine Nucleoside Phosphorylase. 1. Structure-Function Studies
|
|
17726
|
Purine Nucleoside Phosphorylase. 1. Structure-Function Studies
|
|
17727
|
Purine Nucleoside Phosphorylase. 1. Structure-Function Studies
|
|
17728
|
Purine Nucleoside Phosphorylase. 1. Structure-Function Studies
|
|
17729
|
Purine Nucleoside Phosphorylase. 1. Structure-Function Studies
|
|
17730
|
Cysteine synthase of an extremely thermophilic bacterium, Thermus thermophilus HB8
|
|
17731
|
Cysteine synthase of an extremely thermophilic bacterium, Thermus thermophilus HB8
|
|
17732
|
Cysteine synthase of an extremely thermophilic bacterium, Thermus thermophilus HB8
|
|
17733
|
Cysteine synthase of an extremely thermophilic bacterium, Thermus thermophilus HB8
|
|
17734
|
Cysteine synthase of an extremely thermophilic bacterium, Thermus thermophilus HB8
|
|
17735
|
Cysteine synthase of an extremely thermophilic bacterium, Thermus thermophilus HB8
|
|
17736
|
Deletion of the four C-terminal residues of PepC converts an aminopeptidase into an oligopeptidase
|
|
17737
|
Deletion of the four C-terminal residues of PepC converts an aminopeptidase into an oligopeptidase
|
|
17738
|
Deletion of the four C-terminal residues of PepC converts an aminopeptidase into an oligopeptidase
|
|
17739
|
Deletion of the four C-terminal residues of PepC converts an aminopeptidase into an oligopeptidase
|
|
17740
|
tRNA-guanine transglycosylase from Escherichia coli: gross tRNA structural requirements for recognition
|
|
17741
|
Amino acid residues that contribute to substrate specificity of class A beta-lactamase SME-1
|
|
17742
|
Amino acid residues that contribute to substrate specificity of class A beta-lactamase SME-1
|
|
17743
|
Amino acid residues that contribute to substrate specificity of class A beta-lactamase SME-1
|
|
17744
|
Amino acid residues that contribute to substrate specificity of class A beta-lactamase SME-1
|
|
17745
|
Amino acid residues that contribute to substrate specificity of class A beta-lactamase SME-1
|
|
17746
|
Amino acid residues that contribute to substrate specificity of class A beta-lactamase SME-1
|
|
17747
|
Amino acid residues that contribute to substrate specificity of class A beta-lactamase SME-1
|
|
17748
|
Amino acid residues that contribute to substrate specificity of class A beta-lactamase SME-1
|
|
17759
|
Purification and functional characterization of a novel alpha-L-arabinofuranosidase from Bifidobacterium ...
|
|
17760
|
Purification and functional characterization of a novel alpha-L-arabinofuranosidase from Bifidobacterium ...
|
|
17761
|
Purification and functional characterization of a novel alpha-L-arabinofuranosidase from Bifidobacterium ...
|
|
17762
|
Purification and Characterization of an Aminopeptidase from Lactococcus lactis subsp. cremoris AM2
|
|
17763
|
Purification of a putative K+-ATPase from Streptococcus faecalis
|
|
17764
|
Purification of a putative K+-ATPase from Streptococcus faecalis
|
|
17765
|
Functional characterization of OXA-57, a class D beta-lactamase from Burkholderia pseudomallei
|
|
17766
|
Functional characterization of OXA-57, a class D beta-lactamase from Burkholderia pseudomallei
|
|
17767
|
Functional characterization of OXA-57, a class D beta-lactamase from Burkholderia pseudomallei
|
|
17768
|
Functional characterization of OXA-57, a class D beta-lactamase from Burkholderia pseudomallei
|
|
17769
|
Functional characterization of OXA-57, a class D beta-lactamase from Burkholderia pseudomallei
|
|
17770
|
Functional characterization of OXA-57, a class D beta-lactamase from Burkholderia pseudomallei
|
|
17771
|
Functional characterization of OXA-57, a class D beta-lactamase from Burkholderia pseudomallei
|
|
17772
|
Functional characterization of OXA-57, a class D beta-lactamase from Burkholderia pseudomallei
|
|
17773
|
Functional characterization of OXA-57, a class D beta-lactamase from Burkholderia pseudomallei
|
|
17774
|
Functional characterization of OXA-57, a class D beta-lactamase from Burkholderia pseudomallei
|
|
17775
|
Functional characterization of OXA-57, a class D beta-lactamase from Burkholderia pseudomallei
|
|
17776
|
Functional characterization of OXA-57, a class D beta-lactamase from Burkholderia pseudomallei
|
|
17777
|
Functional characterization of OXA-57, a class D beta-lactamase from Burkholderia pseudomallei
|
|
17778
|
Functional characterization of OXA-57, a class D beta-lactamase from Burkholderia pseudomallei
|
|
17779
|
Functional characterization of OXA-57, a class D beta-lactamase from Burkholderia pseudomallei
|
|
17780
|
Functional characterization of OXA-57, a class D beta-lactamase from Burkholderia pseudomallei
|
|
17781
|
Functional characterization of OXA-57, a class D beta-lactamase from Burkholderia pseudomallei
|
|
17782
|
Functional characterization of OXA-57, a class D beta-lactamase from Burkholderia pseudomallei
|
|
17783
|
Functional characterization of OXA-57, a class D beta-lactamase from Burkholderia pseudomallei
|
|
17784
|
Functional characterization of OXA-57, a class D beta-lactamase from Burkholderia pseudomallei
|
|
17785
|
Functional characterization of OXA-57, a class D beta-lactamase from Burkholderia pseudomallei
|
|
17786
|
Functional characterization of OXA-57, a class D beta-lactamase from Burkholderia pseudomallei
|
|
17787
|
Functional characterization of OXA-57, a class D beta-lactamase from Burkholderia pseudomallei
|
|
17788
|
Functional characterization of OXA-57, a class D beta-lactamase from Burkholderia pseudomallei
|
|
17789
|
Functional characterization of OXA-57, a class D beta-lactamase from Burkholderia pseudomallei
|
|
17790
|
A kinetic study of NMC-A beta-lactamase, an Ambler class A carbapenemase also hydrolyzing cephamycins
|
|
17791
|
A kinetic study of NMC-A beta-lactamase, an Ambler class A carbapenemase also hydrolyzing cephamycins
|
|
17792
|
A kinetic study of NMC-A beta-lactamase, an Ambler class A carbapenemase also hydrolyzing cephamycins
|
|
17793
|
A kinetic study of NMC-A beta-lactamase, an Ambler class A carbapenemase also hydrolyzing cephamycins
|
|
17794
|
A kinetic study of NMC-A beta-lactamase, an Ambler class A carbapenemase also hydrolyzing cephamycins
|
|
17795
|
A kinetic study of NMC-A beta-lactamase, an Ambler class A carbapenemase also hydrolyzing cephamycins
|
|
17796
|
A kinetic study of NMC-A beta-lactamase, an Ambler class A carbapenemase also hydrolyzing cephamycins
|
|
17797
|
A kinetic study of NMC-A beta-lactamase, an Ambler class A carbapenemase also hydrolyzing cephamycins
|
|
17798
|
A kinetic study of NMC-A beta-lactamase, an Ambler class A carbapenemase also hydrolyzing cephamycins
|
|
17799
|
A kinetic study of NMC-A beta-lactamase, an Ambler class A carbapenemase also hydrolyzing cephamycins
|
|
17800
|
A kinetic study of NMC-A beta-lactamase, an Ambler class A carbapenemase also hydrolyzing cephamycins
|
|
17801
|
A kinetic study of NMC-A beta-lactamase, an Ambler class A carbapenemase also hydrolyzing cephamycins
|
|
17802
|
A kinetic study of NMC-A beta-lactamase, an Ambler class A carbapenemase also hydrolyzing cephamycins
|
|
17803
|
A kinetic study of NMC-A beta-lactamase, an Ambler class A carbapenemase also hydrolyzing cephamycins
|
|
17804
|
A kinetic study of NMC-A beta-lactamase, an Ambler class A carbapenemase also hydrolyzing cephamycins
|
|
17805
|
A kinetic study of NMC-A beta-lactamase, an Ambler class A carbapenemase also hydrolyzing cephamycins
|
|
17806
|
Mitochondrial malate dehydrogenase from the thermophilic, filamentous fungus Talaromyces emersonii.
|
|
17807
|
Mitochondrial malate dehydrogenase from the thermophilic, filamentous fungus Talaromyces emersonii.
|
|
17808
|
Mitochondrial malate dehydrogenase from the thermophilic, filamentous fungus Talaromyces emersonii.
|
|
17809
|
Mitochondrial malate dehydrogenase from the thermophilic, filamentous fungus Talaromyces emersonii.
|
|
17810
|
Mitochondrial malate dehydrogenase from the thermophilic, filamentous fungus Talaromyces emersonii.
|
|
17811
|
Mitochondrial malate dehydrogenase from the thermophilic, filamentous fungus Talaromyces emersonii.
|
|
17812
|
Mitochondrial malate dehydrogenase from the thermophilic, filamentous fungus Talaromyces emersonii.
|
|
17813
|
Mitochondrial malate dehydrogenase from the thermophilic, filamentous fungus Talaromyces emersonii.
|
|
17814
|
An alpha-proteobacterial type malate dehydrogenase may complement LDH function in Plasmodium falciparum. ...
|
|
17815
|
An alpha-proteobacterial type malate dehydrogenase may complement LDH function in Plasmodium falciparum. ...
|
|
17816
|
An alpha-proteobacterial type malate dehydrogenase may complement LDH function in Plasmodium falciparum. ...
|
|
17817
|
An alpha-proteobacterial type malate dehydrogenase may complement LDH function in Plasmodium falciparum. ...
|
|
17818
|
An alpha-proteobacterial type malate dehydrogenase may complement LDH function in Plasmodium falciparum. ...
|
|
17819
|
An alpha-proteobacterial type malate dehydrogenase may complement LDH function in Plasmodium falciparum. ...
|
|
17820
|
An alpha-proteobacterial type malate dehydrogenase may complement LDH function in Plasmodium falciparum. ...
|
|
17821
|
An alpha-proteobacterial type malate dehydrogenase may complement LDH function in Plasmodium falciparum. ...
|
|
17822
|
An alpha-proteobacterial type malate dehydrogenase may complement LDH function in Plasmodium falciparum. ...
|
|
17823
|
An alpha-proteobacterial type malate dehydrogenase may complement LDH function in Plasmodium falciparum. ...
|
|
17824
|
An alpha-proteobacterial type malate dehydrogenase may complement LDH function in Plasmodium falciparum. ...
|
|
17825
|
An alpha-proteobacterial type malate dehydrogenase may complement LDH function in Plasmodium falciparum. ...
|
|
17826
|
An alpha-proteobacterial type malate dehydrogenase may complement LDH function in Plasmodium falciparum. ...
|
|
17827
|
An alpha-proteobacterial type malate dehydrogenase may complement LDH function in Plasmodium falciparum. ...
|
|
17828
|
Inhibition of yeast phosphoglycerate kinase by lanthanide-ATP complexes
|
|
17829
|
Inhibition of yeast phosphoglycerate kinase by lanthanide-ATP complexes
|
|
17830
|
Inhibition of yeast phosphoglycerate kinase by lanthanide-ATP complexes
|
|
17831
|
Human UDP-glucuronosyltransferase 1A1 is the primary enzyme responsible for the N-glucuronidation of ...
|
|
17832
|
Human UDP-glucuronosyltransferase 1A1 is the primary enzyme responsible for the N-glucuronidation of ...
|
|
17833
|
Human UDP-glucuronosyltransferase 1A1 is the primary enzyme responsible for the N-glucuronidation of ...
|
|
17834
|
Human UDP-glucuronosyltransferase 1A1 is the primary enzyme responsible for the N-glucuronidation of ...
|
|
17835
|
Human UDP-glucuronosyltransferase 1A1 is the primary enzyme responsible for the N-glucuronidation of ...
|
|
17836
|
Human UDP-glucuronosyltransferase 1A1 is the primary enzyme responsible for the N-glucuronidation of ...
|
|
17837
|
Human UDP-glucuronosyltransferase 1A1 is the primary enzyme responsible for the N-glucuronidation of ...
|
|
17838
|
Human UDP-glucuronosyltransferase 1A1 is the primary enzyme responsible for the N-glucuronidation of ...
|
|
17846
|
Glucosephosphate isomerase from Trypanosoma brucei. Cloning and characterization of the gene and analysis of ...
|
|
17847
|
Glucosephosphate isomerase from Trypanosoma brucei. Cloning and characterization of the gene and analysis of ...
|
|
17848
|
Glucosephosphate isomerase from Trypanosoma brucei. Cloning and characterization of the gene and analysis of ...
|
|
17849
|
Glucosephosphate isomerase from Trypanosoma brucei. Cloning and characterization of the gene and analysis of ...
|
|
17850
|
Glucosephosphate isomerase from Trypanosoma brucei. Cloning and characterization of the gene and analysis of ...
|
|
17851
|
Glucosephosphate isomerase from Trypanosoma brucei. Cloning and characterization of the gene and analysis of ...
|
|
17852
|
Glucosephosphate isomerase from Trypanosoma brucei. Cloning and characterization of the gene and analysis of ...
|
|
17853
|
Glucosephosphate isomerase from Trypanosoma brucei. Cloning and characterization of the gene and analysis of ...
|
|
17854
|
Glucosephosphate isomerase from Trypanosoma brucei. Cloning and characterization of the gene and analysis of ...
|
|
17855
|
Glucosephosphate isomerase from Trypanosoma brucei. Cloning and characterization of the gene and analysis of ...
|
|
17856
|
Glucosephosphate isomerase from Trypanosoma brucei. Cloning and characterization of the gene and analysis of ...
|
|
17857
|
Kinetic, spectroscopic and thermodynamic characterization of the Mycobacterium tuberculosis adrenodoxin ...
|
|
17858
|
Kinetic, spectroscopic and thermodynamic characterization of the Mycobacterium tuberculosis adrenodoxin ...
|
|
17859
|
Kinetic, spectroscopic and thermodynamic characterization of the Mycobacterium tuberculosis adrenodoxin ...
|
|
17860
|
Kinetic, spectroscopic and thermodynamic characterization of the Mycobacterium tuberculosis adrenodoxin ...
|
|
17861
|
Kinetic, spectroscopic and thermodynamic characterization of the Mycobacterium tuberculosis adrenodoxin ...
|
|
17862
|
Kinetic, spectroscopic and thermodynamic characterization of the Mycobacterium tuberculosis adrenodoxin ...
|
|
17863
|
Kinetics and Crystal Structure of the Wild-Type and the Engineered Y101F Mutant of Herpes simplex Virus Type 1 ...
|
|
17864
|
Kinetics and Crystal Structure of the Wild-Type and the Engineered Y101F Mutant of Herpes simplex Virus Type 1 ...
|
|
17865
|
Kinetics and Crystal Structure of the Wild-Type and the Engineered Y101F Mutant of Herpes simplex Virus Type 1 ...
|
|
17866
|
Kinetics and Crystal Structure of the Wild-Type and the Engineered Y101F Mutant of Herpes simplex Virus Type 1 ...
|
|
17867
|
Kinetics and Crystal Structure of the Wild-Type and the Engineered Y101F Mutant of Herpes simplex Virus Type 1 ...
|
|
17868
|
Kinetics and Crystal Structure of the Wild-Type and the Engineered Y101F Mutant of Herpes simplex Virus Type 1 ...
|
|
17869
|
Mutagenesis studies of protein farnesyltransferase implicate aspartate beta 352 as a magnesium ligand
|
|
17870
|
Mutagenesis studies of protein farnesyltransferase implicate aspartate beta 352 as a magnesium ligand
|
|
17871
|
Mutagenesis studies of protein farnesyltransferase implicate aspartate beta 352 as a magnesium ligand
|
|
17872
|
Mutagenesis studies of protein farnesyltransferase implicate aspartate beta 352 as a magnesium ligand
|
|
17873
|
Mutagenesis studies of protein farnesyltransferase implicate aspartate beta 352 as a magnesium ligand
|
|
17874
|
Mutagenesis studies of protein farnesyltransferase implicate aspartate beta 352 as a magnesium ligand
|
|
17875
|
Mutagenesis studies of protein farnesyltransferase implicate aspartate beta 352 as a magnesium ligand
|
|
17876
|
Mutagenesis studies of protein farnesyltransferase implicate aspartate beta 352 as a magnesium ligand
|
|
17877
|
Mutagenesis studies of protein farnesyltransferase implicate aspartate beta 352 as a magnesium ligand
|
|
17878
|
Mutagenesis studies of protein farnesyltransferase implicate aspartate beta 352 as a magnesium ligand
|
|
17879
|
Mutagenesis studies of protein farnesyltransferase implicate aspartate beta 352 as a magnesium ligand
|
|
17880
|
Mutagenesis studies of protein farnesyltransferase implicate aspartate beta 352 as a magnesium ligand
|
|
17881
|
Mutagenesis studies of protein farnesyltransferase implicate aspartate beta 352 as a magnesium ligand
|
|
17882
|
Mutagenesis studies of protein farnesyltransferase implicate aspartate beta 352 as a magnesium ligand
|
|
17883
|
Mutagenesis studies of protein farnesyltransferase implicate aspartate beta 352 as a magnesium ligand
|
|
17884
|
Mutagenesis studies of protein farnesyltransferase implicate aspartate beta 352 as a magnesium ligand
|
|
17885
|
Mutagenesis studies of protein farnesyltransferase implicate aspartate beta 352 as a magnesium ligand
|
|
17886
|
Arginine-specific modification of rabbit muscle phosphoglucose isomerase: differences in the inactivation by ...
|
|
17887
|
Arginine-specific modification of rabbit muscle phosphoglucose isomerase: differences in the inactivation by ...
|
|
17888
|
Arginine-specific modification of rabbit muscle phosphoglucose isomerase: differences in the inactivation by ...
|
|
17889
|
Arginine-specific modification of rabbit muscle phosphoglucose isomerase: differences in the inactivation by ...
|
|
17890
|
Arginine-specific modification of rabbit muscle phosphoglucose isomerase: differences in the inactivation by ...
|
|
17891
|
Arginine-specific modification of rabbit muscle phosphoglucose isomerase: differences in the inactivation by ...
|
|
17892
|
Arginine-specific modification of rabbit muscle phosphoglucose isomerase: differences in the inactivation by ...
|
|
17893
|
Arginine-specific modification of rabbit muscle phosphoglucose isomerase: differences in the inactivation by ...
|
|
17894
|
Isolation and characterization of cytosolic alanine aminotransferase isoforms from starved rat liver
|
|
17895
|
Isolation and characterization of cytosolic alanine aminotransferase isoforms from starved rat liver
|
|
17896
|
Isolation and characterization of cytosolic alanine aminotransferase isoforms from starved rat liver
|
|
17897
|
Isolation and characterization of cytosolic alanine aminotransferase isoforms from starved rat liver
|
|
17900
|
Partial purification and kinetic properties of human placental cytosolic aspartate transaminase
|
|
17901
|
Partial purification and kinetic properties of human placental cytosolic aspartate transaminase
|
|
17902
|
Partial purification and kinetic properties of human placental cytosolic aspartate transaminase
|
|
17903
|
UMP kinase from Streptococcus pneumoniae: evidence for co-operative ATP binding and allosteric regulation
|
|
17904
|
UMP kinase from Streptococcus pneumoniae: evidence for co-operative ATP binding and allosteric regulation
|
|
17905
|
UMP kinase from Streptococcus pneumoniae: evidence for co-operative ATP binding and allosteric regulation
|
|
17906
|
UMP kinase from Streptococcus pneumoniae: evidence for co-operative ATP binding and allosteric regulation
|
|
17907
|
UMP kinase from Streptococcus pneumoniae: evidence for co-operative ATP binding and allosteric regulation
|
|
17908
|
UMP kinase from Streptococcus pneumoniae: evidence for co-operative ATP binding and allosteric regulation
|
|
17909
|
UMP kinase from Streptococcus pneumoniae: evidence for co-operative ATP binding and allosteric regulation
|
|
17910
|
UMP kinase from Streptococcus pneumoniae: evidence for co-operative ATP binding and allosteric regulation
|
|
17911
|
UMP kinase from Streptococcus pneumoniae: evidence for co-operative ATP binding and allosteric regulation
|
|
17912
|
UMP kinase from Streptococcus pneumoniae: evidence for co-operative ATP binding and allosteric regulation
|
|
17913
|
UMP kinase from Streptococcus pneumoniae: evidence for co-operative ATP binding and allosteric regulation
|
|
17914
|
UMP kinase from Streptococcus pneumoniae: evidence for co-operative ATP binding and allosteric regulation
|
|
17915
|
UMP kinase from Streptococcus pneumoniae: evidence for co-operative ATP binding and allosteric regulation
|
|
17916
|
UMP kinase from Streptococcus pneumoniae: evidence for co-operative ATP binding and allosteric regulation
|
|
17917
|
UMP kinase from Streptococcus pneumoniae: evidence for co-operative ATP binding and allosteric regulation
|
|
17918
|
UMP kinase from Streptococcus pneumoniae: evidence for co-operative ATP binding and allosteric regulation
|
|
17919
|
UMP kinase from Streptococcus pneumoniae: evidence for co-operative ATP binding and allosteric regulation
|
|
17920
|
UMP kinase from Streptococcus pneumoniae: evidence for co-operative ATP binding and allosteric regulation
|
|
17921
|
UMP kinase from Streptococcus pneumoniae: evidence for co-operative ATP binding and allosteric regulation
|
|
17922
|
UMP kinase from Streptococcus pneumoniae: evidence for co-operative ATP binding and allosteric regulation
|
|
17923
|
UMP kinase from Streptococcus pneumoniae: evidence for co-operative ATP binding and allosteric regulation
|
|
17924
|
UMP kinase from Streptococcus pneumoniae: evidence for co-operative ATP binding and allosteric regulation
|
|
17925
|
UMP kinase from Streptococcus pneumoniae: evidence for co-operative ATP binding and allosteric regulation
|
|
17926
|
UMP kinase from Streptococcus pneumoniae: evidence for co-operative ATP binding and allosteric regulation
|
|
17927
|
UMP kinase from Streptococcus pneumoniae: evidence for co-operative ATP binding and allosteric regulation
|
|
17928
|
UMP kinase from Streptococcus pneumoniae: evidence for co-operative ATP binding and allosteric regulation
|
|
17929
|
UMP kinase from Streptococcus pneumoniae: evidence for co-operative ATP binding and allosteric regulation
|
|
17930
|
UMP kinase from Streptococcus pneumoniae: evidence for co-operative ATP binding and allosteric regulation
|
|
17931
|
UMP kinase from Streptococcus pneumoniae: evidence for co-operative ATP binding and allosteric regulation
|
|
17932
|
UMP kinase from Streptococcus pneumoniae: evidence for co-operative ATP binding and allosteric regulation
|
|
17933
|
UMP kinase from Streptococcus pneumoniae: evidence for co-operative ATP binding and allosteric regulation
|
|
17934
|
UMP kinase from Streptococcus pneumoniae: evidence for co-operative ATP binding and allosteric regulation
|
|
17935
|
UMP kinase from Streptococcus pneumoniae: evidence for co-operative ATP binding and allosteric regulation
|
|
17936
|
Cloning, expression, and characterization of a human inosine triphosphate pyrophosphatase encoded by the itpa ...
|
|
17937
|
Cloning, expression, and characterization of a human inosine triphosphate pyrophosphatase encoded by the itpa ...
|
|
17938
|
Cloning, expression, and characterization of a human inosine triphosphate pyrophosphatase encoded by the itpa ...
|
|
17939
|
Human fatty acid synthase: properties and molecular cloning
|
|
17940
|
Human fatty acid synthase: properties and molecular cloning
|
|
17941
|
Human fatty acid synthase: properties and molecular cloning
|
|
17942
|
Human fatty acid synthase: properties and molecular cloning
|
|
17968
|
The comparative enzymology of lactic dehydrogenases. 3. Properties of the H4 and M4 enzymes from a number of ...
|
|
17969
|
The comparative enzymology of lactic dehydrogenases. 3. Properties of the H4 and M4 enzymes from a number of ...
|
|
17970
|
The comparative enzymology of lactic dehydrogenases. 3. Properties of the H4 and M4 enzymes from a number of ...
|
|
17971
|
The comparative enzymology of lactic dehydrogenases. 3. Properties of the H4 and M4 enzymes from a number of ...
|
|
17972
|
The comparative enzymology of lactic dehydrogenases. 3. Properties of the H4 and M4 enzymes from a number of ...
|
|
17973
|
The comparative enzymology of lactic dehydrogenases. 3. Properties of the H4 and M4 enzymes from a number of ...
|
|
17974
|
The comparative enzymology of lactic dehydrogenases. 3. Properties of the H4 and M4 enzymes from a number of ...
|
|
17975
|
The comparative enzymology of lactic dehydrogenases. 3. Properties of the H4 and M4 enzymes from a number of ...
|
|
17976
|
The comparative enzymology of lactic dehydrogenases. 3. Properties of the H4 and M4 enzymes from a number of ...
|
|
17977
|
The comparative enzymology of lactic dehydrogenases. 3. Properties of the H4 and M4 enzymes from a number of ...
|
|
17978
|
The comparative enzymology of lactic dehydrogenases. 3. Properties of the H4 and M4 enzymes from a number of ...
|
|
17979
|
The comparative enzymology of lactic dehydrogenases. 3. Properties of the H4 and M4 enzymes from a number of ...
|
|
17980
|
The comparative enzymology of lactic dehydrogenases. 3. Properties of the H4 and M4 enzymes from a number of ...
|
|
17981
|
The comparative enzymology of lactic dehydrogenases. 3. Properties of the H4 and M4 enzymes from a number of ...
|
|
17982
|
The comparative enzymology of lactic dehydrogenases. 3. Properties of the H4 and M4 enzymes from a number of ...
|
|
17983
|
The comparative enzymology of lactic dehydrogenases. 3. Properties of the H4 and M4 enzymes from a number of ...
|
|
17984
|
The comparative enzymology of lactic dehydrogenases. 3. Properties of the H4 and M4 enzymes from a number of ...
|
|
17985
|
The comparative enzymology of lactic dehydrogenases. 3. Properties of the H4 and M4 enzymes from a number of ...
|
|
17986
|
The comparative enzymology of lactic dehydrogenases. 3. Properties of the H4 and M4 enzymes from a number of ...
|
|
17987
|
The comparative enzymology of lactic dehydrogenases. 3. Properties of the H4 and M4 enzymes from a number of ...
|
|
17988
|
The comparative enzymology of lactic dehydrogenases. 3. Properties of the H4 and M4 enzymes from a number of ...
|
|
17989
|
The comparative enzymology of lactic dehydrogenases. 3. Properties of the H4 and M4 enzymes from a number of ...
|
|
17990
|
The comparative enzymology of lactic dehydrogenases. 3. Properties of the H4 and M4 enzymes from a number of ...
|
|
17991
|
The comparative enzymology of lactic dehydrogenases. 3. Properties of the H4 and M4 enzymes from a number of ...
|
|
17992
|
The comparative enzymology of lactic dehydrogenases. 3. Properties of the H4 and M4 enzymes from a number of ...
|
|
17993
|
The comparative enzymology of lactic dehydrogenases. 3. Properties of the H4 and M4 enzymes from a number of ...
|
|
17994
|
The comparative enzymology of lactic dehydrogenases. 3. Properties of the H4 and M4 enzymes from a number of ...
|
|
17995
|
The comparative enzymology of lactic dehydrogenases. 3. Properties of the H4 and M4 enzymes from a number of ...
|
|
17996
|
Hydrolytic metabolism of pyrethroids by human and other mammalian carboxylesterases
|
|
17997
|
Hydrolytic metabolism of pyrethroids by human and other mammalian carboxylesterases
|
|
17998
|
Hydrolytic metabolism of pyrethroids by human and other mammalian carboxylesterases
|
|
17999
|
Hydrolytic metabolism of pyrethroids by human and other mammalian carboxylesterases
|
|
18000
|
Hydrolytic metabolism of pyrethroids by human and other mammalian carboxylesterases
|




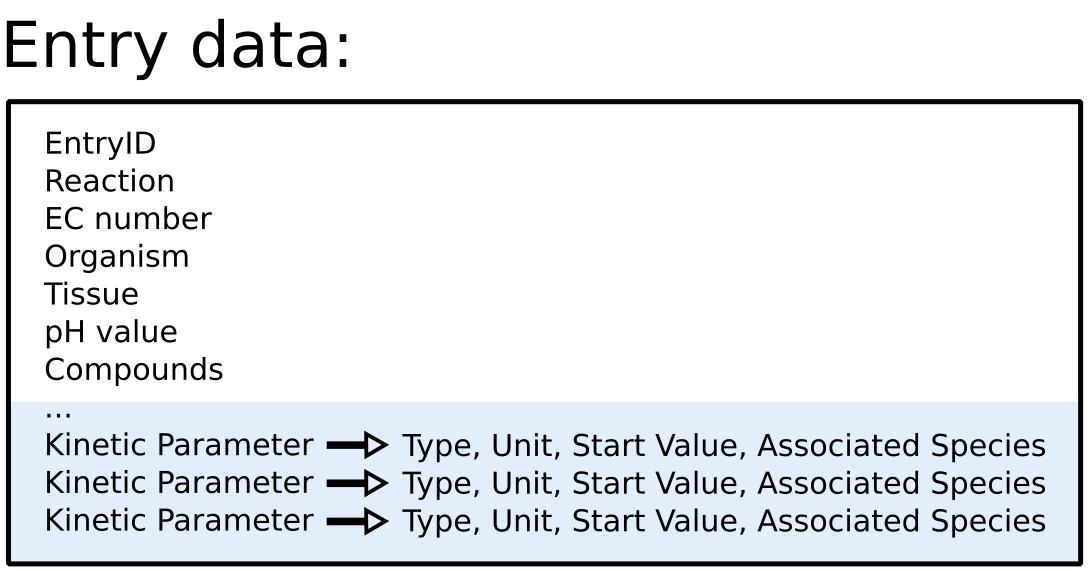
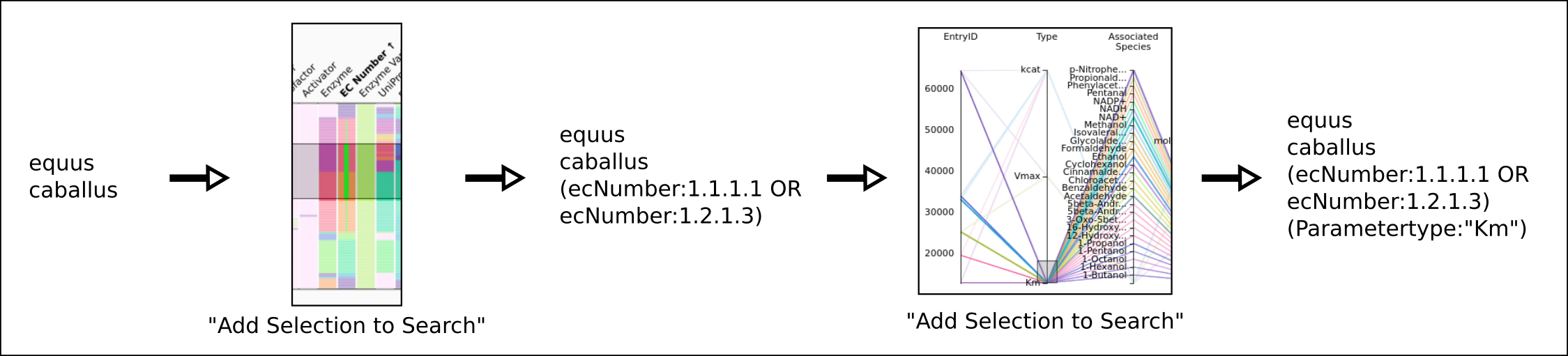 If the current search query is a refinement of the previous query "Use Previous Query (Go Back)" button can be used to perform the previous query again. Only one automatic step back is possible, but the query can be manually manipulated if desired.
If the current search query is a refinement of the previous query "Use Previous Query (Go Back)" button can be used to perform the previous query again. Only one automatic step back is possible, but the query can be manually manipulated if desired.