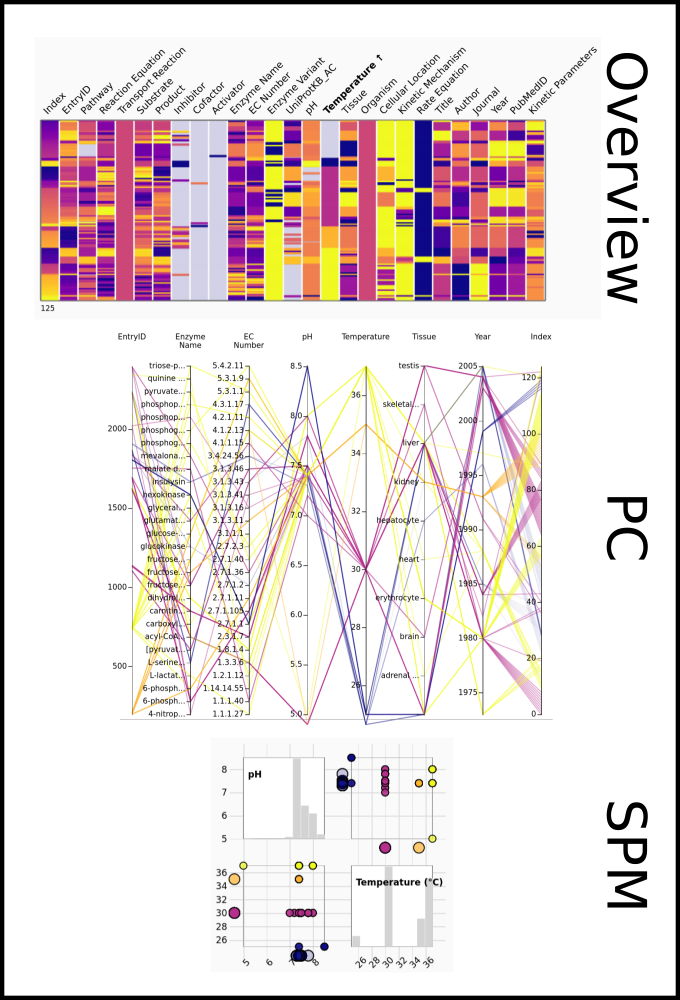
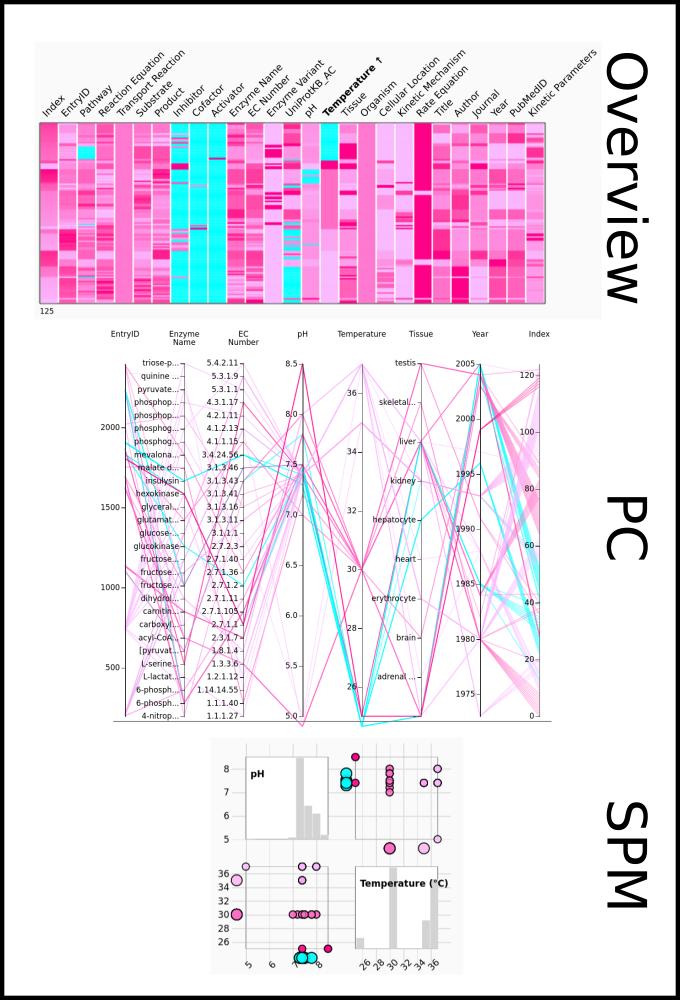
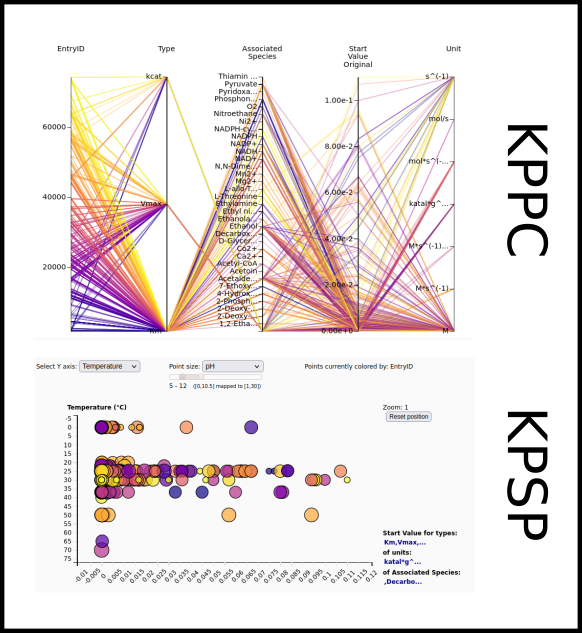
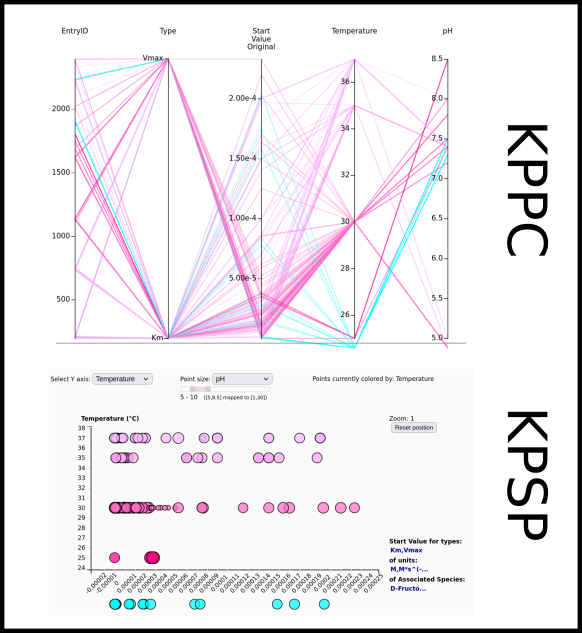
To improve SABIO-RK data interoperability, semantic markup was added to web pages as described and defined by
This structured information makes it easier to discover, collate and analyse our data.
|
21001
|
Separation and properties of NAD+- and NADP+-dependent glyceraldehyde-3-phosphate dehydrogenases from ...
|
|
21002
|
Separation and properties of NAD+- and NADP+-dependent glyceraldehyde-3-phosphate dehydrogenases from ...
|
|
21003
|
Separation and properties of NAD+- and NADP+-dependent glyceraldehyde-3-phosphate dehydrogenases from ...
|
|
21004
|
Separation and properties of NAD+- and NADP+-dependent glyceraldehyde-3-phosphate dehydrogenases from ...
|
|
21005
|
Separation and properties of NAD+- and NADP+-dependent glyceraldehyde-3-phosphate dehydrogenases from ...
|
|
21006
|
Separation and properties of NAD+- and NADP+-dependent glyceraldehyde-3-phosphate dehydrogenases from ...
|
|
21007
|
Separation and properties of NAD+- and NADP+-dependent glyceraldehyde-3-phosphate dehydrogenases from ...
|
|
21008
|
Separation and properties of NAD+- and NADP+-dependent glyceraldehyde-3-phosphate dehydrogenases from ...
|
|
21009
|
Separation and properties of NAD+- and NADP+-dependent glyceraldehyde-3-phosphate dehydrogenases from ...
|
|
21010
|
Separation and properties of NAD+- and NADP+-dependent glyceraldehyde-3-phosphate dehydrogenases from ...
|
|
21011
|
Separation and properties of NAD+- and NADP+-dependent glyceraldehyde-3-phosphate dehydrogenases from ...
|
|
21012
|
Separation and properties of NAD+- and NADP+-dependent glyceraldehyde-3-phosphate dehydrogenases from ...
|
|
21013
|
Separation and properties of NAD+- and NADP+-dependent glyceraldehyde-3-phosphate dehydrogenases from ...
|
|
21014
|
Arginine 165/arginine 277 pair in (S)-mandelate dehydrogenase from Pseudomonas putida: role in catalysis and ...
|
|
21015
|
Arginine 165/arginine 277 pair in (S)-mandelate dehydrogenase from Pseudomonas putida: role in catalysis and ...
|
|
21016
|
Arginine 165/arginine 277 pair in (S)-mandelate dehydrogenase from Pseudomonas putida: role in catalysis and ...
|
|
21017
|
Arginine 165/arginine 277 pair in (S)-mandelate dehydrogenase from Pseudomonas putida: role in catalysis and ...
|
|
21018
|
Arginine 165/arginine 277 pair in (S)-mandelate dehydrogenase from Pseudomonas putida: role in catalysis and ...
|
|
21019
|
Arginine 165/arginine 277 pair in (S)-mandelate dehydrogenase from Pseudomonas putida: role in catalysis and ...
|
|
21020
|
Arginine 165/arginine 277 pair in (S)-mandelate dehydrogenase from Pseudomonas putida: role in catalysis and ...
|
|
21021
|
Arginine 165/arginine 277 pair in (S)-mandelate dehydrogenase from Pseudomonas putida: role in catalysis and ...
|
|
21022
|
Arginine 165/arginine 277 pair in (S)-mandelate dehydrogenase from Pseudomonas putida: role in catalysis and ...
|
|
21023
|
Arginine 165/arginine 277 pair in (S)-mandelate dehydrogenase from Pseudomonas putida: role in catalysis and ...
|
|
21024
|
Arginine 165/arginine 277 pair in (S)-mandelate dehydrogenase from Pseudomonas putida: role in catalysis and ...
|
|
21025
|
Arginine 165/arginine 277 pair in (S)-mandelate dehydrogenase from Pseudomonas putida: role in catalysis and ...
|
|
21026
|
Arginine 165/arginine 277 pair in (S)-mandelate dehydrogenase from Pseudomonas putida: role in catalysis and ...
|
|
21027
|
Arginine 165/arginine 277 pair in (S)-mandelate dehydrogenase from Pseudomonas putida: role in catalysis and ...
|
|
21028
|
Arginine 165/arginine 277 pair in (S)-mandelate dehydrogenase from Pseudomonas putida: role in catalysis and ...
|
|
21029
|
Arginine 165/arginine 277 pair in (S)-mandelate dehydrogenase from Pseudomonas putida: role in catalysis and ...
|
|
21030
|
Arginine 165/arginine 277 pair in (S)-mandelate dehydrogenase from Pseudomonas putida: role in catalysis and ...
|
|
21031
|
Arginine 165/arginine 277 pair in (S)-mandelate dehydrogenase from Pseudomonas putida: role in catalysis and ...
|
|
21032
|
Isozymic nature of spore coat-associated alanine racemase of Bacillus subtilis
|
|
21033
|
Isozymic nature of spore coat-associated alanine racemase of Bacillus subtilis
|
|
21034
|
Isozymic nature of spore coat-associated alanine racemase of Bacillus subtilis
|
|
21035
|
Isozymic nature of spore coat-associated alanine racemase of Bacillus subtilis
|
|
21036
|
Isozymic nature of spore coat-associated alanine racemase of Bacillus subtilis
|
|
21037
|
Isozymic nature of spore coat-associated alanine racemase of Bacillus subtilis
|
|
21038
|
Isozymic nature of spore coat-associated alanine racemase of Bacillus subtilis
|
|
21039
|
Isozymic nature of spore coat-associated alanine racemase of Bacillus subtilis
|
|
21040
|
Isozymic nature of spore coat-associated alanine racemase of Bacillus subtilis
|
|
21041
|
Isozymic nature of spore coat-associated alanine racemase of Bacillus subtilis
|
|
21042
|
Isozymic nature of spore coat-associated alanine racemase of Bacillus subtilis
|
|
21043
|
Isozymic nature of spore coat-associated alanine racemase of Bacillus subtilis
|
|
21044
|
Isozymic nature of spore coat-associated alanine racemase of Bacillus subtilis
|
|
21045
|
Isozymic nature of spore coat-associated alanine racemase of Bacillus subtilis
|
|
21046
|
Isozymic nature of spore coat-associated alanine racemase of Bacillus subtilis
|
|
21047
|
Isozymic nature of spore coat-associated alanine racemase of Bacillus subtilis
|
|
21048
|
Isozymic nature of spore coat-associated alanine racemase of Bacillus subtilis
|
|
21049
|
Isozymic nature of spore coat-associated alanine racemase of Bacillus subtilis
|
|
21050
|
Isozymic nature of spore coat-associated alanine racemase of Bacillus subtilis
|
|
21051
|
Role of residues 104, 164, 166, 238 and 240 in the substrate profile of PER-1 beta-lactamase hydrolysing ...
|
|
21052
|
Role of residues 104, 164, 166, 238 and 240 in the substrate profile of PER-1 beta-lactamase hydrolysing ...
|
|
21053
|
Role of residues 104, 164, 166, 238 and 240 in the substrate profile of PER-1 beta-lactamase hydrolysing ...
|
|
21054
|
Role of residues 104, 164, 166, 238 and 240 in the substrate profile of PER-1 beta-lactamase hydrolysing ...
|
|
21055
|
Role of residues 104, 164, 166, 238 and 240 in the substrate profile of PER-1 beta-lactamase hydrolysing ...
|
|
21056
|
Role of residues 104, 164, 166, 238 and 240 in the substrate profile of PER-1 beta-lactamase hydrolysing ...
|
|
21057
|
Role of residues 104, 164, 166, 238 and 240 in the substrate profile of PER-1 beta-lactamase hydrolysing ...
|
|
21058
|
Role of residues 104, 164, 166, 238 and 240 in the substrate profile of PER-1 beta-lactamase hydrolysing ...
|
|
21059
|
Role of residues 104, 164, 166, 238 and 240 in the substrate profile of PER-1 beta-lactamase hydrolysing ...
|
|
21060
|
Role of residues 104, 164, 166, 238 and 240 in the substrate profile of PER-1 beta-lactamase hydrolysing ...
|
|
21061
|
Role of residues 104, 164, 166, 238 and 240 in the substrate profile of PER-1 beta-lactamase hydrolysing ...
|
|
21062
|
Role of residues 104, 164, 166, 238 and 240 in the substrate profile of PER-1 beta-lactamase hydrolysing ...
|
|
21063
|
Role of residues 104, 164, 166, 238 and 240 in the substrate profile of PER-1 beta-lactamase hydrolysing ...
|
|
21064
|
Role of residues 104, 164, 166, 238 and 240 in the substrate profile of PER-1 beta-lactamase hydrolysing ...
|
|
21065
|
Role of residues 104, 164, 166, 238 and 240 in the substrate profile of PER-1 beta-lactamase hydrolysing ...
|
|
21066
|
Role of residues 104, 164, 166, 238 and 240 in the substrate profile of PER-1 beta-lactamase hydrolysing ...
|
|
21067
|
Role of residues 104, 164, 166, 238 and 240 in the substrate profile of PER-1 beta-lactamase hydrolysing ...
|
|
21068
|
Role of residues 104, 164, 166, 238 and 240 in the substrate profile of PER-1 beta-lactamase hydrolysing ...
|
|
21069
|
Role of residues 104, 164, 166, 238 and 240 in the substrate profile of PER-1 beta-lactamase hydrolysing ...
|
|
21070
|
Role of residues 104, 164, 166, 238 and 240 in the substrate profile of PER-1 beta-lactamase hydrolysing ...
|
|
21071
|
Role of residues 104, 164, 166, 238 and 240 in the substrate profile of PER-1 beta-lactamase hydrolysing ...
|
|
21072
|
Role of residues 104, 164, 166, 238 and 240 in the substrate profile of PER-1 beta-lactamase hydrolysing ...
|
|
21073
|
Role of residues 104, 164, 166, 238 and 240 in the substrate profile of PER-1 beta-lactamase hydrolysing ...
|
|
21074
|
Role of residues 104, 164, 166, 238 and 240 in the substrate profile of PER-1 beta-lactamase hydrolysing ...
|
|
21075
|
Role of residues 104, 164, 166, 238 and 240 in the substrate profile of PER-1 beta-lactamase hydrolysing ...
|
|
21076
|
Role of residues 104, 164, 166, 238 and 240 in the substrate profile of PER-1 beta-lactamase hydrolysing ...
|
|
21077
|
Role of residues 104, 164, 166, 238 and 240 in the substrate profile of PER-1 beta-lactamase hydrolysing ...
|
|
21078
|
Role of residues 104, 164, 166, 238 and 240 in the substrate profile of PER-1 beta-lactamase hydrolysing ...
|
|
21079
|
Role of residues 104, 164, 166, 238 and 240 in the substrate profile of PER-1 beta-lactamase hydrolysing ...
|
|
21080
|
Role of residues 104, 164, 166, 238 and 240 in the substrate profile of PER-1 beta-lactamase hydrolysing ...
|
|
21081
|
Role of residues 104, 164, 166, 238 and 240 in the substrate profile of PER-1 beta-lactamase hydrolysing ...
|
|
21082
|
Role of residues 104, 164, 166, 238 and 240 in the substrate profile of PER-1 beta-lactamase hydrolysing ...
|
|
21083
|
Role of residues 104, 164, 166, 238 and 240 in the substrate profile of PER-1 beta-lactamase hydrolysing ...
|
|
21084
|
Role of residues 104, 164, 166, 238 and 240 in the substrate profile of PER-1 beta-lactamase hydrolysing ...
|
|
21085
|
Role of residues 104, 164, 166, 238 and 240 in the substrate profile of PER-1 beta-lactamase hydrolysing ...
|
|
21086
|
Role of residues 104, 164, 166, 238 and 240 in the substrate profile of PER-1 beta-lactamase hydrolysing ...
|
|
21087
|
Role of residues 104, 164, 166, 238 and 240 in the substrate profile of PER-1 beta-lactamase hydrolysing ...
|
|
21088
|
Role of residues 104, 164, 166, 238 and 240 in the substrate profile of PER-1 beta-lactamase hydrolysing ...
|
|
21089
|
Role of residues 104, 164, 166, 238 and 240 in the substrate profile of PER-1 beta-lactamase hydrolysing ...
|
|
21090
|
Role of residues 104, 164, 166, 238 and 240 in the substrate profile of PER-1 beta-lactamase hydrolysing ...
|
|
21091
|
Role of residues 104, 164, 166, 238 and 240 in the substrate profile of PER-1 beta-lactamase hydrolysing ...
|
|
21092
|
Role of residues 104, 164, 166, 238 and 240 in the substrate profile of PER-1 beta-lactamase hydrolysing ...
|
|
21093
|
Role of residues 104, 164, 166, 238 and 240 in the substrate profile of PER-1 beta-lactamase hydrolysing ...
|
|
21094
|
Role of residues 104, 164, 166, 238 and 240 in the substrate profile of PER-1 beta-lactamase hydrolysing ...
|
|
21095
|
Hibernation impact on the catalytic activities of the mitochondrial D-3-hydroxybutyrate dehydrogenase in liver ...
|
|
21096
|
Hibernation impact on the catalytic activities of the mitochondrial D-3-hydroxybutyrate dehydrogenase in liver ...
|
|
21097
|
Hibernation impact on the catalytic activities of the mitochondrial D-3-hydroxybutyrate dehydrogenase in liver ...
|
|
21098
|
Hibernation impact on the catalytic activities of the mitochondrial D-3-hydroxybutyrate dehydrogenase in liver ...
|
|
21099
|
Hibernation impact on the catalytic activities of the mitochondrial D-3-hydroxybutyrate dehydrogenase in liver ...
|
|
21100
|
Hibernation impact on the catalytic activities of the mitochondrial D-3-hydroxybutyrate dehydrogenase in liver ...
|
|
21101
|
Hibernation impact on the catalytic activities of the mitochondrial D-3-hydroxybutyrate dehydrogenase in liver ...
|
|
21102
|
Hibernation impact on the catalytic activities of the mitochondrial D-3-hydroxybutyrate dehydrogenase in liver ...
|
|
21103
|
Hibernation impact on the catalytic activities of the mitochondrial D-3-hydroxybutyrate dehydrogenase in liver ...
|
|
21104
|
Hibernation impact on the catalytic activities of the mitochondrial D-3-hydroxybutyrate dehydrogenase in liver ...
|
|
21105
|
Hibernation impact on the catalytic activities of the mitochondrial D-3-hydroxybutyrate dehydrogenase in liver ...
|
|
21106
|
Hibernation impact on the catalytic activities of the mitochondrial D-3-hydroxybutyrate dehydrogenase in liver ...
|
|
21107
|
Hibernation impact on the catalytic activities of the mitochondrial D-3-hydroxybutyrate dehydrogenase in liver ...
|
|
21108
|
Hibernation impact on the catalytic activities of the mitochondrial D-3-hydroxybutyrate dehydrogenase in liver ...
|
|
21109
|
Hibernation impact on the catalytic activities of the mitochondrial D-3-hydroxybutyrate dehydrogenase in liver ...
|
|
21110
|
Hibernation impact on the catalytic activities of the mitochondrial D-3-hydroxybutyrate dehydrogenase in liver ...
|
|
21111
|
Hibernation impact on the catalytic activities of the mitochondrial D-3-hydroxybutyrate dehydrogenase in liver ...
|
|
21112
|
Hibernation impact on the catalytic activities of the mitochondrial D-3-hydroxybutyrate dehydrogenase in liver ...
|
|
21113
|
Hibernation impact on the catalytic activities of the mitochondrial D-3-hydroxybutyrate dehydrogenase in liver ...
|
|
21114
|
Hibernation impact on the catalytic activities of the mitochondrial D-3-hydroxybutyrate dehydrogenase in liver ...
|
|
21115
|
Hibernation impact on the catalytic activities of the mitochondrial D-3-hydroxybutyrate dehydrogenase in liver ...
|
|
21116
|
Hibernation impact on the catalytic activities of the mitochondrial D-3-hydroxybutyrate dehydrogenase in liver ...
|
|
21117
|
Hibernation impact on the catalytic activities of the mitochondrial D-3-hydroxybutyrate dehydrogenase in liver ...
|
|
21118
|
Hibernation impact on the catalytic activities of the mitochondrial D-3-hydroxybutyrate dehydrogenase in liver ...
|
|
21119
|
Purification and properties of thyroidal UDP-glucose-4-epimerase
|
|
21120
|
Purification and properties of thyroidal UDP-glucose-4-epimerase
|
|
21121
|
Purification and properties of thyroidal UDP-glucose-4-epimerase
|
|
21122
|
Purification and characterization of the 4-aminobutyrate--2,ketoglutarate transaminase from mouse brain
|
|
21123
|
Purification and characterization of the 4-aminobutyrate--2,ketoglutarate transaminase from mouse brain
|
|
21124
|
Studies of phospholipid-requiring bacterial enzymes. 3. Purification and properties of uridine diphosphate ...
|
|
21125
|
Studies of phospholipid-requiring bacterial enzymes. 3. Purification and properties of uridine diphosphate ...
|
|
21126
|
Essential role of histidine 20 in the catalytic mechanism of Escherichia coli peptidyl-tRNA hydrolase
|
|
21127
|
Essential role of histidine 20 in the catalytic mechanism of Escherichia coli peptidyl-tRNA hydrolase
|
|
21128
|
Essential role of histidine 20 in the catalytic mechanism of Escherichia coli peptidyl-tRNA hydrolase
|
|
21129
|
Essential role of histidine 20 in the catalytic mechanism of Escherichia coli peptidyl-tRNA hydrolase
|
|
21130
|
Essential role of histidine 20 in the catalytic mechanism of Escherichia coli peptidyl-tRNA hydrolase
|
|
21131
|
Essential role of histidine 20 in the catalytic mechanism of Escherichia coli peptidyl-tRNA hydrolase
|
|
21132
|
Essential role of histidine 20 in the catalytic mechanism of Escherichia coli peptidyl-tRNA hydrolase
|
|
21133
|
Essential role of histidine 20 in the catalytic mechanism of Escherichia coli peptidyl-tRNA hydrolase
|
|
21134
|
Essential role of histidine 20 in the catalytic mechanism of Escherichia coli peptidyl-tRNA hydrolase
|
|
21135
|
Essential role of histidine 20 in the catalytic mechanism of Escherichia coli peptidyl-tRNA hydrolase
|
|
21136
|
Essential role of histidine 20 in the catalytic mechanism of Escherichia coli peptidyl-tRNA hydrolase
|
|
21137
|
The contribution of adjacent subunits to the active sites of 3-D-Phosphoglycerate Dehydrogenase
|
|
21138
|
The contribution of adjacent subunits to the active sites of 3-D-Phosphoglycerate Dehydrogenase
|
|
21139
|
The contribution of adjacent subunits to the active sites of 3-D-Phosphoglycerate Dehydrogenase
|
|
21140
|
The contribution of adjacent subunits to the active sites of 3-D-Phosphoglycerate Dehydrogenase
|
|
21141
|
The contribution of adjacent subunits to the active sites of 3-D-Phosphoglycerate Dehydrogenase
|
|
21142
|
The contribution of adjacent subunits to the active sites of 3-D-Phosphoglycerate Dehydrogenase
|
|
21143
|
The contribution of adjacent subunits to the active sites of 3-D-Phosphoglycerate Dehydrogenase
|
|
21144
|
The contribution of adjacent subunits to the active sites of 3-D-Phosphoglycerate Dehydrogenase
|
|
21145
|
The contribution of adjacent subunits to the active sites of 3-D-Phosphoglycerate Dehydrogenase
|
|
21146
|
The contribution of adjacent subunits to the active sites of 3-D-Phosphoglycerate Dehydrogenase
|
|
21147
|
The contribution of adjacent subunits to the active sites of 3-D-Phosphoglycerate Dehydrogenase
|
|
21148
|
The contribution of adjacent subunits to the active sites of 3-D-Phosphoglycerate Dehydrogenase
|
|
21149
|
The contribution of adjacent subunits to the active sites of 3-D-Phosphoglycerate Dehydrogenase
|
|
21150
|
The contribution of adjacent subunits to the active sites of 3-D-Phosphoglycerate Dehydrogenase
|
|
21151
|
The contribution of adjacent subunits to the active sites of 3-D-Phosphoglycerate Dehydrogenase
|
|
21152
|
The contribution of adjacent subunits to the active sites of 3-D-Phosphoglycerate Dehydrogenase
|
|
21153
|
The contribution of adjacent subunits to the active sites of 3-D-Phosphoglycerate Dehydrogenase
|
|
21154
|
The contribution of adjacent subunits to the active sites of 3-D-Phosphoglycerate Dehydrogenase
|
|
21155
|
The contribution of adjacent subunits to the active sites of 3-D-Phosphoglycerate Dehydrogenase
|
|
21156
|
The contribution of adjacent subunits to the active sites of 3-D-Phosphoglycerate Dehydrogenase
|
|
21157
|
The contribution of adjacent subunits to the active sites of 3-D-Phosphoglycerate Dehydrogenase
|
|
21158
|
The contribution of adjacent subunits to the active sites of 3-D-Phosphoglycerate Dehydrogenase
|
|
21159
|
The contribution of adjacent subunits to the active sites of 3-D-Phosphoglycerate Dehydrogenase
|
|
21160
|
The contribution of adjacent subunits to the active sites of 3-D-Phosphoglycerate Dehydrogenase
|
|
21161
|
The contribution of adjacent subunits to the active sites of 3-D-Phosphoglycerate Dehydrogenase
|
|
21162
|
The contribution of adjacent subunits to the active sites of 3-D-Phosphoglycerate Dehydrogenase
|
|
21163
|
The contribution of adjacent subunits to the active sites of 3-D-Phosphoglycerate Dehydrogenase
|
|
21164
|
The contribution of adjacent subunits to the active sites of 3-D-Phosphoglycerate Dehydrogenase
|
|
21165
|
The contribution of adjacent subunits to the active sites of 3-D-Phosphoglycerate Dehydrogenase
|
|
21166
|
The contribution of adjacent subunits to the active sites of 3-D-Phosphoglycerate Dehydrogenase
|
|
21167
|
Site-directed mutagenesis of beta-lactamase I: role of Glu-166
|
|
21168
|
Site-directed mutagenesis of beta-lactamase I: role of Glu-166
|
|
21169
|
Site-directed mutagenesis of beta-lactamase I: role of Glu-166
|
|
21170
|
Site-directed mutagenesis of beta-lactamase I: role of Glu-166
|
|
21171
|
Site-directed mutagenesis of beta-lactamase I: role of Glu-166
|
|
21172
|
Site-directed mutagenesis of beta-lactamase I: role of Glu-166
|
|
21173
|
Site-directed mutagenesis of beta-lactamase I: role of Glu-166
|
|
21174
|
Site-directed mutagenesis of beta-lactamase I: role of Glu-166
|
|
21175
|
Site-directed mutagenesis of beta-lactamase I: role of Glu-166
|
|
21176
|
Site-directed mutagenesis of beta-lactamase I: role of Glu-166
|
|
21177
|
Site-directed mutagenesis of beta-lactamase I: role of Glu-166
|
|
21178
|
Site-directed mutagenesis of beta-lactamase I: role of Glu-166
|
|
21179
|
Site-directed mutagenesis of beta-lactamase I: role of Glu-166
|
|
21180
|
Site-directed mutagenesis of beta-lactamase I: role of Glu-166
|
|
21181
|
Site-directed mutagenesis of beta-lactamase I: role of Glu-166
|
|
21182
|
Site-directed mutagenesis of beta-lactamase I: role of Glu-166
|
|
21183
|
Site-directed mutagenesis of beta-lactamase I: role of Glu-166
|
|
21184
|
Site-directed mutagenesis of beta-lactamase I: role of Glu-166
|
|
21185
|
Site-directed mutagenesis of beta-lactamase I: role of Glu-166
|
|
21186
|
Site-directed mutagenesis of beta-lactamase I: role of Glu-166
|
|
21187
|
Properties of mutant SHV-5 beta-lactamases constructed by substitution of isoleucine or valine for methionine ...
|
|
21188
|
Properties of mutant SHV-5 beta-lactamases constructed by substitution of isoleucine or valine for methionine ...
|
|
21189
|
Properties of mutant SHV-5 beta-lactamases constructed by substitution of isoleucine or valine for methionine ...
|
|
21190
|
Properties of mutant SHV-5 beta-lactamases constructed by substitution of isoleucine or valine for methionine ...
|
|
21191
|
Properties of mutant SHV-5 beta-lactamases constructed by substitution of isoleucine or valine for methionine ...
|
|
21192
|
Properties of mutant SHV-5 beta-lactamases constructed by substitution of isoleucine or valine for methionine ...
|
|
21193
|
Properties of mutant SHV-5 beta-lactamases constructed by substitution of isoleucine or valine for methionine ...
|
|
21194
|
Properties of mutant SHV-5 beta-lactamases constructed by substitution of isoleucine or valine for methionine ...
|
|
21195
|
Properties of mutant SHV-5 beta-lactamases constructed by substitution of isoleucine or valine for methionine ...
|
|
21196
|
Properties of mutant SHV-5 beta-lactamases constructed by substitution of isoleucine or valine for methionine ...
|
|
21197
|
Properties of mutant SHV-5 beta-lactamases constructed by substitution of isoleucine or valine for methionine ...
|
|
21198
|
Properties of mutant SHV-5 beta-lactamases constructed by substitution of isoleucine or valine for methionine ...
|
|
21199
|
Properties of mutant SHV-5 beta-lactamases constructed by substitution of isoleucine or valine for methionine ...
|
|
21200
|
Properties of mutant SHV-5 beta-lactamases constructed by substitution of isoleucine or valine for methionine ...
|
|
21201
|
Properties of mutant SHV-5 beta-lactamases constructed by substitution of isoleucine or valine for methionine ...
|
|
21202
|
Properties of mutant SHV-5 beta-lactamases constructed by substitution of isoleucine or valine for methionine ...
|
|
21203
|
Properties of mutant SHV-5 beta-lactamases constructed by substitution of isoleucine or valine for methionine ...
|
|
21204
|
Properties of mutant SHV-5 beta-lactamases constructed by substitution of isoleucine or valine for methionine ...
|
|
21205
|
Properties of mutant SHV-5 beta-lactamases constructed by substitution of isoleucine or valine for methionine ...
|
|
21206
|
Properties of mutant SHV-5 beta-lactamases constructed by substitution of isoleucine or valine for methionine ...
|
|
21207
|
Properties of mutant SHV-5 beta-lactamases constructed by substitution of isoleucine or valine for methionine ...
|
|
21215
|
Use of site-directed mutagenesis to identify residues specific for each reaction catalyzed by Chorismate ...
|
|
21216
|
Use of site-directed mutagenesis to identify residues specific for each reaction catalyzed by Chorismate ...
|
|
21217
|
Use of site-directed mutagenesis to identify residues specific for each reaction catalyzed by Chorismate ...
|
|
21218
|
Use of site-directed mutagenesis to identify residues specific for each reaction catalyzed by Chorismate ...
|
|
21219
|
Use of site-directed mutagenesis to identify residues specific for each reaction catalyzed by Chorismate ...
|
|
21220
|
Use of site-directed mutagenesis to identify residues specific for each reaction catalyzed by Chorismate ...
|
|
21221
|
Use of site-directed mutagenesis to identify residues specific for each reaction catalyzed by Chorismate ...
|
|
21222
|
Use of site-directed mutagenesis to identify residues specific for each reaction catalyzed by Chorismate ...
|
|
21223
|
Use of site-directed mutagenesis to identify residues specific for each reaction catalyzed by Chorismate ...
|
|
21224
|
Use of site-directed mutagenesis to identify residues specific for each reaction catalyzed by Chorismate ...
|
|
21225
|
Use of site-directed mutagenesis to identify residues specific for each reaction catalyzed by Chorismate ...
|
|
21226
|
Use of site-directed mutagenesis to identify residues specific for each reaction catalyzed by Chorismate ...
|
|
21227
|
Use of site-directed mutagenesis to identify residues specific for each reaction catalyzed by Chorismate ...
|
|
21228
|
Use of site-directed mutagenesis to identify residues specific for each reaction catalyzed by Chorismate ...
|
|
21229
|
Use of site-directed mutagenesis to identify residues specific for each reaction catalyzed by Chorismate ...
|
|
21230
|
Use of site-directed mutagenesis to identify residues specific for each reaction catalyzed by Chorismate ...
|
|
21231
|
Use of site-directed mutagenesis to identify residues specific for each reaction catalyzed by Chorismate ...
|
|
21232
|
Use of site-directed mutagenesis to identify residues specific for each reaction catalyzed by Chorismate ...
|
|
21233
|
Use of site-directed mutagenesis to identify residues specific for each reaction catalyzed by Chorismate ...
|
|
21234
|
Use of site-directed mutagenesis to identify residues specific for each reaction catalyzed by Chorismate ...
|
|
21235
|
Use of site-directed mutagenesis to identify residues specific for each reaction catalyzed by Chorismate ...
|
|
21236
|
Use of site-directed mutagenesis to identify residues specific for each reaction catalyzed by Chorismate ...
|
|
21237
|
Use of site-directed mutagenesis to identify residues specific for each reaction catalyzed by Chorismate ...
|
|
21238
|
Use of site-directed mutagenesis to identify residues specific for each reaction catalyzed by Chorismate ...
|
|
21239
|
Use of site-directed mutagenesis to identify residues specific for each reaction catalyzed by Chorismate ...
|
|
21240
|
Use of site-directed mutagenesis to identify residues specific for each reaction catalyzed by Chorismate ...
|
|
21241
|
Use of site-directed mutagenesis to identify residues specific for each reaction catalyzed by Chorismate ...
|
|
21242
|
Use of site-directed mutagenesis to identify residues specific for each reaction catalyzed by Chorismate ...
|
|
21243
|
Use of site-directed mutagenesis to identify residues specific for each reaction catalyzed by Chorismate ...
|
|
21244
|
Use of site-directed mutagenesis to identify residues specific for each reaction catalyzed by Chorismate ...
|
|
21245
|
Human milk bile salt-activated lipase. Further characterization and kinetic studies
|
|
21246
|
Human milk bile salt-activated lipase. Further characterization and kinetic studies
|
|
21247
|
Human milk bile salt-activated lipase. Further characterization and kinetic studies
|
|
21248
|
Human milk bile salt-activated lipase. Further characterization and kinetic studies
|
|
21249
|
Human milk bile salt-activated lipase. Further characterization and kinetic studies
|
|
21250
|
Human milk bile salt-activated lipase. Further characterization and kinetic studies
|
|
21251
|
Human milk bile salt-activated lipase. Further characterization and kinetic studies
|
|
21252
|
Human milk bile salt-activated lipase. Further characterization and kinetic studies
|
|
21253
|
Human milk bile salt-activated lipase. Further characterization and kinetic studies
|
|
21254
|
Human milk bile salt-activated lipase. Further characterization and kinetic studies
|
|
21255
|
3-phosphoglycerate kinase from rabbit sceletal muscle and yeast
|
|
21256
|
3-phosphoglycerate kinase from rabbit sceletal muscle and yeast
|
|
21257
|
3-phosphoglycerate kinase from rabbit sceletal muscle and yeast
|
|
21258
|
3-phosphoglycerate kinase from rabbit sceletal muscle and yeast
|
|
21259
|
3-phosphoglycerate kinase from rabbit sceletal muscle and yeast
|
|
21260
|
3-phosphoglycerate kinase from rabbit sceletal muscle and yeast
|
|
21261
|
3-phosphoglycerate kinase from rabbit sceletal muscle and yeast
|
|
21262
|
3-phosphoglycerate kinase from rabbit sceletal muscle and yeast
|
|
21263
|
Mitochondrial aldehyde reductase: identification and characterization in rat liver and kidney cortex
|
|
21264
|
Mitochondrial aldehyde reductase: identification and characterization in rat liver and kidney cortex
|
|
21265
|
Mitochondrial aldehyde reductase: identification and characterization in rat liver and kidney cortex
|
|
21266
|
Mitochondrial aldehyde reductase: identification and characterization in rat liver and kidney cortex
|
|
21267
|
Mitochondrial aldehyde reductase: identification and characterization in rat liver and kidney cortex
|
|
21268
|
Mitochondrial aldehyde reductase: identification and characterization in rat liver and kidney cortex
|
|
21269
|
Mitochondrial aldehyde reductase: identification and characterization in rat liver and kidney cortex
|
|
21270
|
Mitochondrial aldehyde reductase: identification and characterization in rat liver and kidney cortex
|
|
21271
|
Mitochondrial aldehyde reductase: identification and characterization in rat liver and kidney cortex
|
|
21272
|
Mitochondrial aldehyde reductase: identification and characterization in rat liver and kidney cortex
|
|
21273
|
Mitochondrial aldehyde reductase: identification and characterization in rat liver and kidney cortex
|
|
21274
|
Mitochondrial aldehyde reductase: identification and characterization in rat liver and kidney cortex
|
|
21275
|
Location of the coenzyme binding site in the porcine mitochondrial NADP-dependent isocitrate dehydrogenase
|
|
21276
|
Location of the coenzyme binding site in the porcine mitochondrial NADP-dependent isocitrate dehydrogenase
|
|
21277
|
Location of the coenzyme binding site in the porcine mitochondrial NADP-dependent isocitrate dehydrogenase
|
|
21278
|
Location of the coenzyme binding site in the porcine mitochondrial NADP-dependent isocitrate dehydrogenase
|
|
21279
|
Location of the coenzyme binding site in the porcine mitochondrial NADP-dependent isocitrate dehydrogenase
|
|
21280
|
Location of the coenzyme binding site in the porcine mitochondrial NADP-dependent isocitrate dehydrogenase
|
|
21281
|
Location of the coenzyme binding site in the porcine mitochondrial NADP-dependent isocitrate dehydrogenase
|
|
21282
|
Location of the coenzyme binding site in the porcine mitochondrial NADP-dependent isocitrate dehydrogenase
|
|
21283
|
Location of the coenzyme binding site in the porcine mitochondrial NADP-dependent isocitrate dehydrogenase
|
|
21284
|
Location of the coenzyme binding site in the porcine mitochondrial NADP-dependent isocitrate dehydrogenase
|
|
21285
|
Location of the coenzyme binding site in the porcine mitochondrial NADP-dependent isocitrate dehydrogenase
|
|
21286
|
Location of the coenzyme binding site in the porcine mitochondrial NADP-dependent isocitrate dehydrogenase
|
|
21287
|
Location of the coenzyme binding site in the porcine mitochondrial NADP-dependent isocitrate dehydrogenase
|
|
21288
|
Location of the coenzyme binding site in the porcine mitochondrial NADP-dependent isocitrate dehydrogenase
|
|
21289
|
Location of the coenzyme binding site in the porcine mitochondrial NADP-dependent isocitrate dehydrogenase
|
|
21290
|
Location of the coenzyme binding site in the porcine mitochondrial NADP-dependent isocitrate dehydrogenase
|
|
21291
|
Location of the coenzyme binding site in the porcine mitochondrial NADP-dependent isocitrate dehydrogenase
|
|
21292
|
Location of the coenzyme binding site in the porcine mitochondrial NADP-dependent isocitrate dehydrogenase
|
|
21293
|
Location of the coenzyme binding site in the porcine mitochondrial NADP-dependent isocitrate dehydrogenase
|
|
21294
|
Location of the coenzyme binding site in the porcine mitochondrial NADP-dependent isocitrate dehydrogenase
|
|
21295
|
Location of the coenzyme binding site in the porcine mitochondrial NADP-dependent isocitrate dehydrogenase
|
|
21296
|
Location of the coenzyme binding site in the porcine mitochondrial NADP-dependent isocitrate dehydrogenase
|
|
21297
|
Location of the coenzyme binding site in the porcine mitochondrial NADP-dependent isocitrate dehydrogenase
|
|
21298
|
Location of the coenzyme binding site in the porcine mitochondrial NADP-dependent isocitrate dehydrogenase
|
|
21299
|
The presence of 2-keto-3-deoxygluconic acid and oxoaldehyde dehydrogenase activity in human erythrocytes
|
|
21300
|
The presence of 2-keto-3-deoxygluconic acid and oxoaldehyde dehydrogenase activity in human erythrocytes
|
|
21301
|
The presence of 2-keto-3-deoxygluconic acid and oxoaldehyde dehydrogenase activity in human erythrocytes
|
|
21302
|
Stereo-specific substrate recognition by phosphatidylinositol phosphate kinases is swapped by changing a ...
|
|
21303
|
Stereo-specific substrate recognition by phosphatidylinositol phosphate kinases is swapped by changing a ...
|
|
21304
|
Stereo-specific substrate recognition by phosphatidylinositol phosphate kinases is swapped by changing a ...
|
|
21305
|
Stereo-specific substrate recognition by phosphatidylinositol phosphate kinases is swapped by changing a ...
|
|
21306
|
Stereo-specific substrate recognition by phosphatidylinositol phosphate kinases is swapped by changing a ...
|
|
21307
|
Stereo-specific substrate recognition by phosphatidylinositol phosphate kinases is swapped by changing a ...
|
|
21308
|
Stereo-specific substrate recognition by phosphatidylinositol phosphate kinases is swapped by changing a ...
|
|
21309
|
Stereo-specific substrate recognition by phosphatidylinositol phosphate kinases is swapped by changing a ...
|
|
21310
|
Stereo-specific substrate recognition by phosphatidylinositol phosphate kinases is swapped by changing a ...
|
|
21311
|
Stereo-specific substrate recognition by phosphatidylinositol phosphate kinases is swapped by changing a ...
|
|
21312
|
Stereo-specific substrate recognition by phosphatidylinositol phosphate kinases is swapped by changing a ...
|
|
21313
|
Purification, characterization, and biosynthesis of human acid ceramidase
|
|
21314
|
Purification, characterization, and biosynthesis of human acid ceramidase
|
|
21315
|
Purification, characterization, and biosynthesis of human acid ceramidase
|
|
21316
|
Purification, characterization, and biosynthesis of human acid ceramidase
|
|
21317
|
Purification, characterization, and biosynthesis of human acid ceramidase
|
|
21318
|
Purification, characterization, and biosynthesis of human acid ceramidase
|
|
21319
|
Purification, characterization, and biosynthesis of human acid ceramidase
|
|
21320
|
Purification, characterization, and biosynthesis of human acid ceramidase
|
|
21321
|
Purification, characterization, and biosynthesis of human acid ceramidase
|
|
21322
|
Short-chain 3-hydroxy-2-methylacyl-CoA dehydrogenase from rat liver: purification and characterization of a ...
|
|
21323
|
Short-chain 3-hydroxy-2-methylacyl-CoA dehydrogenase from rat liver: purification and characterization of a ...
|
|
21324
|
Short-chain 3-hydroxy-2-methylacyl-CoA dehydrogenase from rat liver: purification and characterization of a ...
|
|
21325
|
Fructose metabolizing enzymes in the rat liver and metabolic parameters: Interactions between dietary copper, ...
|
|
21326
|
Fructose metabolizing enzymes in the rat liver and metabolic parameters: Interactions between dietary copper, ...
|
|
21327
|
Fructose metabolizing enzymes in the rat liver and metabolic parameters: Interactions between dietary copper, ...
|
|
21328
|
Fructose metabolizing enzymes in the rat liver and metabolic parameters: Interactions between dietary copper, ...
|
|
21329
|
Fructose metabolizing enzymes in the rat liver and metabolic parameters: Interactions between dietary copper, ...
|
|
21330
|
Fructose metabolizing enzymes in the rat liver and metabolic parameters: Interactions between dietary copper, ...
|
|
21331
|
Fructose metabolizing enzymes in the rat liver and metabolic parameters: Interactions between dietary copper, ...
|
|
21332
|
Fructose metabolizing enzymes in the rat liver and metabolic parameters: Interactions between dietary copper, ...
|
|
21333
|
Fructose metabolizing enzymes in the rat liver and metabolic parameters: Interactions between dietary copper, ...
|
|
21334
|
Fructose metabolizing enzymes in the rat liver and metabolic parameters: Interactions between dietary copper, ...
|
|
21335
|
Fructose metabolizing enzymes in the rat liver and metabolic parameters: Interactions between dietary copper, ...
|
|
21336
|
Fructose metabolizing enzymes in the rat liver and metabolic parameters: Interactions between dietary copper, ...
|
|
21337
|
Fructose metabolizing enzymes in the rat liver and metabolic parameters: Interactions between dietary copper, ...
|
|
21338
|
Fructose metabolizing enzymes in the rat liver and metabolic parameters: Interactions between dietary copper, ...
|
|
21339
|
Fructose metabolizing enzymes in the rat liver and metabolic parameters: Interactions between dietary copper, ...
|
|
21340
|
Fructose metabolizing enzymes in the rat liver and metabolic parameters: Interactions between dietary copper, ...
|
|
21341
|
Fructose metabolizing enzymes in the rat liver and metabolic parameters: Interactions between dietary copper, ...
|
|
21342
|
Fructose metabolizing enzymes in the rat liver and metabolic parameters: Interactions between dietary copper, ...
|
|
21343
|
Fructose metabolizing enzymes in the rat liver and metabolic parameters: Interactions between dietary copper, ...
|
|
21344
|
Fructose metabolizing enzymes in the rat liver and metabolic parameters: Interactions between dietary copper, ...
|
|
21345
|
Fructose metabolizing enzymes in the rat liver and metabolic parameters: Interactions between dietary copper, ...
|
|
21346
|
Fructose metabolizing enzymes in the rat liver and metabolic parameters: Interactions between dietary copper, ...
|
|
21347
|
Fructose metabolizing enzymes in the rat liver and metabolic parameters: Interactions between dietary copper, ...
|
|
21348
|
Fructose metabolizing enzymes in the rat liver and metabolic parameters: Interactions between dietary copper, ...
|
|
21349
|
Fructose metabolizing enzymes in the rat liver and metabolic parameters: Interactions between dietary copper, ...
|
|
21350
|
Fructose metabolizing enzymes in the rat liver and metabolic parameters: Interactions between dietary copper, ...
|
|
21351
|
Fructose metabolizing enzymes in the rat liver and metabolic parameters: Interactions between dietary copper, ...
|
|
21352
|
Fructose metabolizing enzymes in the rat liver and metabolic parameters: Interactions between dietary copper, ...
|
|
21353
|
Fructose metabolizing enzymes in the rat liver and metabolic parameters: Interactions between dietary copper, ...
|
|
21354
|
Fructose metabolizing enzymes in the rat liver and metabolic parameters: Interactions between dietary copper, ...
|
|
21355
|
Fructose metabolizing enzymes in the rat liver and metabolic parameters: Interactions between dietary copper, ...
|
|
21356
|
Fructose metabolizing enzymes in the rat liver and metabolic parameters: Interactions between dietary copper, ...
|
|
21357
|
Fructose metabolizing enzymes in the rat liver and metabolic parameters: Interactions between dietary copper, ...
|
|
21358
|
Fructose metabolizing enzymes in the rat liver and metabolic parameters: Interactions between dietary copper, ...
|
|
21359
|
Fructose metabolizing enzymes in the rat liver and metabolic parameters: Interactions between dietary copper, ...
|
|
21360
|
Fructose metabolizing enzymes in the rat liver and metabolic parameters: Interactions between dietary copper, ...
|
|
21361
|
Fructose metabolizing enzymes in the rat liver and metabolic parameters: Interactions between dietary copper, ...
|
|
21362
|
Fructose metabolizing enzymes in the rat liver and metabolic parameters: Interactions between dietary copper, ...
|
|
21363
|
Fructose metabolizing enzymes in the rat liver and metabolic parameters: Interactions between dietary copper, ...
|
|
21364
|
Fructose metabolizing enzymes in the rat liver and metabolic parameters: Interactions between dietary copper, ...
|
|
21365
|
Characterization of an in vivo hormonally regulated phosphodiesterase 3 (PDE3) associated with a liver ...
|
|
21366
|
Characterization of an in vivo hormonally regulated phosphodiesterase 3 (PDE3) associated with a liver ...
|
|
21367
|
Characterization of an in vivo hormonally regulated phosphodiesterase 3 (PDE3) associated with a liver ...
|
|
21368
|
Characterization of an in vivo hormonally regulated phosphodiesterase 3 (PDE3) associated with a liver ...
|
|
21369
|
Characterization of an in vivo hormonally regulated phosphodiesterase 3 (PDE3) associated with a liver ...
|
|
21370
|
Characterization of an in vivo hormonally regulated phosphodiesterase 3 (PDE3) associated with a liver ...
|
|
21371
|
Characterization of an in vivo hormonally regulated phosphodiesterase 3 (PDE3) associated with a liver ...
|
|
21372
|
Characterization of an in vivo hormonally regulated phosphodiesterase 3 (PDE3) associated with a liver ...
|
|
21373
|
Characterization of an in vivo hormonally regulated phosphodiesterase 3 (PDE3) associated with a liver ...
|
|
21374
|
Characterization of an in vivo hormonally regulated phosphodiesterase 3 (PDE3) associated with a liver ...
|
|
21375
|
Characterization of an in vivo hormonally regulated phosphodiesterase 3 (PDE3) associated with a liver ...
|
|
21376
|
Characterization of an in vivo hormonally regulated phosphodiesterase 3 (PDE3) associated with a liver ...
|
|
21377
|
Calcium sensitive isocitrate and 2-oxoglutarate dehydrogenase activities in rat liver and AS-30D hepatoma ...
|
|
21378
|
Calcium sensitive isocitrate and 2-oxoglutarate dehydrogenase activities in rat liver and AS-30D hepatoma ...
|
|
21379
|
Calcium sensitive isocitrate and 2-oxoglutarate dehydrogenase activities in rat liver and AS-30D hepatoma ...
|
|
21380
|
Calcium sensitive isocitrate and 2-oxoglutarate dehydrogenase activities in rat liver and AS-30D hepatoma ...
|
|
21381
|
Calcium sensitive isocitrate and 2-oxoglutarate dehydrogenase activities in rat liver and AS-30D hepatoma ...
|
|
21382
|
Calcium sensitive isocitrate and 2-oxoglutarate dehydrogenase activities in rat liver and AS-30D hepatoma ...
|
|
21383
|
Calcium sensitive isocitrate and 2-oxoglutarate dehydrogenase activities in rat liver and AS-30D hepatoma ...
|
|
21384
|
Calcium sensitive isocitrate and 2-oxoglutarate dehydrogenase activities in rat liver and AS-30D hepatoma ...
|
|
21385
|
Calcium sensitive isocitrate and 2-oxoglutarate dehydrogenase activities in rat liver and AS-30D hepatoma ...
|
|
21386
|
Calcium sensitive isocitrate and 2-oxoglutarate dehydrogenase activities in rat liver and AS-30D hepatoma ...
|
|
21387
|
Calcium sensitive isocitrate and 2-oxoglutarate dehydrogenase activities in rat liver and AS-30D hepatoma ...
|
|
21388
|
Calcium sensitive isocitrate and 2-oxoglutarate dehydrogenase activities in rat liver and AS-30D hepatoma ...
|
|
21389
|
D-glycerate kinase deficiency as a cause of D-glyceric aciduria
|
|
21390
|
D-glycerate kinase deficiency as a cause of D-glyceric aciduria
|
|
21391
|
D-glycerate kinase deficiency as a cause of D-glyceric aciduria
|
|
21392
|
D-glycerate kinase deficiency as a cause of D-glyceric aciduria
|
|
21393
|
D-glycerate kinase deficiency as a cause of D-glyceric aciduria
|
|
21394
|
D-glycerate kinase deficiency as a cause of D-glyceric aciduria
|
|
21395
|
D-glycerate kinase deficiency as a cause of D-glyceric aciduria
|
|
21396
|
D-glycerate kinase deficiency as a cause of D-glyceric aciduria
|
|
21397
|
D-glycerate kinase deficiency as a cause of D-glyceric aciduria
|
|
21398
|
D-glycerate kinase deficiency as a cause of D-glyceric aciduria
|
|
21399
|
Characterization of cytosolic malic enzyme in human tumor cells
|
|
21400
|
Characterization of cytosolic malic enzyme in human tumor cells
|
|
21401
|
Characterization of cytosolic malic enzyme in human tumor cells
|
|
21402
|
Characterization of cytosolic malic enzyme in human tumor cells
|
|
21403
|
Characterization of cytosolic malic enzyme in human tumor cells
|
|
21404
|
Characterization of cytosolic malic enzyme in human tumor cells
|
|
21405
|
Human NAD(+)-dependent mitochondrial malic enzyme. cDNA cloning, primary structure, and expression in ...
|
|
21406
|
Human NAD(+)-dependent mitochondrial malic enzyme. cDNA cloning, primary structure, and expression in ...
|
|
21407
|
Human NAD(+)-dependent mitochondrial malic enzyme. cDNA cloning, primary structure, and expression in ...
|
|
21408
|
Biosynthesis of cyclic 2,3-diphosphoglycerate. Isolation and characterization of 2-phosphoglycerate kinase and ...
|
|
21409
|
Biosynthesis of cyclic 2,3-diphosphoglycerate. Isolation and characterization of 2-phosphoglycerate kinase and ...
|
|
21410
|
Biosynthesis of cyclic 2,3-diphosphoglycerate. Isolation and characterization of 2-phosphoglycerate kinase and ...
|
|
21411
|
Biosynthesis of cyclic 2,3-diphosphoglycerate. Isolation and characterization of 2-phosphoglycerate kinase and ...
|
|
21412
|
Site-directed mutagenesis of histidine 62 in the `basic patch` region of yeast phosphoglycerate kinase
|
|
21413
|
Site-directed mutagenesis of histidine 62 in the `basic patch` region of yeast phosphoglycerate kinase
|
|
21414
|
The role of the C-terminal lysine in the hinge bending mechanism of yeast phosphoglycerate kinase
|
|
21415
|
The role of the C-terminal lysine in the hinge bending mechanism of yeast phosphoglycerate kinase
|
|
21416
|
The role of the C-terminal lysine in the hinge bending mechanism of yeast phosphoglycerate kinase
|
|
21417
|
The role of the C-terminal lysine in the hinge bending mechanism of yeast phosphoglycerate kinase
|
|
21418
|
Enzyme kinetic parameters of the fluorescent ATP analogue, 2-aminopurine triphosphate
|
|
21419
|
Enzyme kinetic parameters of the fluorescent ATP analogue, 2-aminopurine triphosphate
|
|
21420
|
Enzyme kinetic parameters of the fluorescent ATP analogue, 2-aminopurine triphosphate
|
|
21421
|
Enzyme kinetic parameters of the fluorescent ATP analogue, 2-aminopurine triphosphate
|
|
21422
|
Enzyme kinetic parameters of the fluorescent ATP analogue, 2-aminopurine triphosphate
|
|
21423
|
Enzyme kinetic parameters of the fluorescent ATP analogue, 2-aminopurine triphosphate
|
|
21424
|
Enzyme kinetic parameters of the fluorescent ATP analogue, 2-aminopurine triphosphate
|
|
21425
|
Enzyme kinetic parameters of the fluorescent ATP analogue, 2-aminopurine triphosphate
|
|
21426
|
Enzyme kinetic parameters of the fluorescent ATP analogue, 2-aminopurine triphosphate
|
|
21427
|
Enzyme kinetic parameters of the fluorescent ATP analogue, 2-aminopurine triphosphate
|
|
21428
|
Enzyme kinetic parameters of the fluorescent ATP analogue, 2-aminopurine triphosphate
|
|
21429
|
Enzyme kinetic parameters of the fluorescent ATP analogue, 2-aminopurine triphosphate
|
|
21430
|
Enzyme kinetic parameters of the fluorescent ATP analogue, 2-aminopurine triphosphate
|
|
21431
|
Enzyme kinetic parameters of the fluorescent ATP analogue, 2-aminopurine triphosphate
|
|
21432
|
Enzyme kinetic parameters of the fluorescent ATP analogue, 2-aminopurine triphosphate
|
|
21433
|
Enzyme kinetic parameters of the fluorescent ATP analogue, 2-aminopurine triphosphate
|
|
21434
|
Enzyme kinetic parameters of the fluorescent ATP analogue, 2-aminopurine triphosphate
|
|
21435
|
Equilibrium and kinetic studies of the pyruvic kinase reaction
|
|
21436
|
Equilibrium and kinetic studies of the pyruvic kinase reaction
|
|
21437
|
Equilibrium and kinetic studies of the pyruvic kinase reaction
|
|
21438
|
Equilibrium and kinetic studies of the pyruvic kinase reaction
|
|
21439
|
Equilibrium and kinetic studies of the pyruvic kinase reaction
|
|
21440
|
Equilibrium and kinetic studies of the pyruvic kinase reaction
|
|
21441
|
Equilibrium and kinetic studies of the pyruvic kinase reaction
|
|
21442
|
Equilibrium and kinetic studies of the pyruvic kinase reaction
|
|
21443
|
Equilibrium and kinetic studies of the pyruvic kinase reaction
|
|
21444
|
Equilibrium and kinetic studies of the pyruvic kinase reaction
|
|
21445
|
Equilibrium and kinetic studies of the pyruvic kinase reaction
|
|
21446
|
Equilibrium and kinetic studies of the pyruvic kinase reaction
|
|
21447
|
Equilibrium and kinetic studies of the pyruvic kinase reaction
|
|
21448
|
Equilibrium and kinetic studies of the pyruvic kinase reaction
|
|
21449
|
Equilibrium and kinetic studies of the pyruvic kinase reaction
|
|
21450
|
Equilibrium and kinetic studies of the pyruvic kinase reaction
|
|
21451
|
A soluble dihydrofolate reductase from human placenta: purification and properties
|
|
21452
|
A soluble dihydrofolate reductase from human placenta: purification and properties
|
|
21453
|
A soluble dihydrofolate reductase from human placenta: purification and properties
|
|
21454
|
A soluble dihydrofolate reductase from human placenta: purification and properties
|
|
21455
|
A soluble dihydrofolate reductase from human placenta: purification and properties
|
|
21456
|
A soluble dihydrofolate reductase from human placenta: purification and properties
|
|
21457
|
A soluble dihydrofolate reductase from human placenta: purification and properties
|
|
21458
|
A soluble dihydrofolate reductase from human placenta: purification and properties
|
|
21459
|
A soluble dihydrofolate reductase from human placenta: purification and properties
|
|
21460
|
A soluble dihydrofolate reductase from human placenta: purification and properties
|
|
21461
|
A soluble dihydrofolate reductase from human placenta: purification and properties
|
|
21462
|
A soluble dihydrofolate reductase from human placenta: purification and properties
|
|
21463
|
A soluble dihydrofolate reductase from human placenta: purification and properties
|
|
21464
|
A soluble dihydrofolate reductase from human placenta: purification and properties
|
|
21465
|
A soluble dihydrofolate reductase from human placenta: purification and properties
|
|
21466
|
A soluble dihydrofolate reductase from human placenta: purification and properties
|
|
21467
|
A soluble dihydrofolate reductase from human placenta: purification and properties
|
|
21468
|
A soluble dihydrofolate reductase from human placenta: purification and properties
|
|
21469
|
A soluble dihydrofolate reductase from human placenta: purification and properties
|
|
21470
|
A soluble dihydrofolate reductase from human placenta: purification and properties
|
|
21471
|
A soluble dihydrofolate reductase from human placenta: purification and properties
|
|
21472
|
A soluble dihydrofolate reductase from human placenta: purification and properties
|
|
21473
|
A soluble dihydrofolate reductase from human placenta: purification and properties
|
|
21474
|
A soluble dihydrofolate reductase from human placenta: purification and properties
|
|
21475
|
A soluble dihydrofolate reductase from human placenta: purification and properties
|
|
21476
|
A soluble dihydrofolate reductase from human placenta: purification and properties
|
|
21477
|
A soluble dihydrofolate reductase from human placenta: purification and properties
|
|
21478
|
A soluble dihydrofolate reductase from human placenta: purification and properties
|
|
21479
|
Comparative studies of the rat and pigeon liver fatty acid synthetases
|
|
21480
|
Modulation of the interaction between aldolase and glycerol-phosphate dehydrogenase by fructose phosphates
|
|
21481
|
Modulation of the interaction between aldolase and glycerol-phosphate dehydrogenase by fructose phosphates
|
|
21482
|
Modulation of the interaction between aldolase and glycerol-phosphate dehydrogenase by fructose phosphates
|
|
21483
|
Modulation of the interaction between aldolase and glycerol-phosphate dehydrogenase by fructose phosphates
|
|
21484
|
Modulation of the interaction between aldolase and glycerol-phosphate dehydrogenase by fructose phosphates
|
|
21485
|
Structural basis for the alteration of coenzyme specificity in a malate dehydrogenase mutant
|
|
21486
|
Structural basis for the alteration of coenzyme specificity in a malate dehydrogenase mutant
|
|
21487
|
Structural basis for the alteration of coenzyme specificity in a malate dehydrogenase mutant
|
|
21488
|
Structural basis for the alteration of coenzyme specificity in a malate dehydrogenase mutant
|
|
21489
|
Structural basis for the alteration of coenzyme specificity in a malate dehydrogenase mutant
|
|
21490
|
Structural basis for the alteration of coenzyme specificity in a malate dehydrogenase mutant
|
|
21491
|
Structural basis for the alteration of coenzyme specificity in a malate dehydrogenase mutant
|
|
21492
|
Structural basis for the alteration of coenzyme specificity in a malate dehydrogenase mutant
|
|
21493
|
Structural basis for the alteration of coenzyme specificity in a malate dehydrogenase mutant
|
|
21494
|
Structural basis for the alteration of coenzyme specificity in a malate dehydrogenase mutant
|
|
21495
|
Structural basis for the alteration of coenzyme specificity in a malate dehydrogenase mutant
|
|
21496
|
Structural basis for the alteration of coenzyme specificity in a malate dehydrogenase mutant
|
|
21497
|
Structural basis for the alteration of coenzyme specificity in a malate dehydrogenase mutant
|
|
21498
|
Structural basis for the alteration of coenzyme specificity in a malate dehydrogenase mutant
|
|
21499
|
Structural basis for the alteration of coenzyme specificity in a malate dehydrogenase mutant
|
|
21500
|
Structural basis for the alteration of coenzyme specificity in a malate dehydrogenase mutant
|
|
21501
|
Structural basis for the alteration of coenzyme specificity in a malate dehydrogenase mutant
|
|
21502
|
Structural basis for the alteration of coenzyme specificity in a malate dehydrogenase mutant
|
|
21503
|
Structural basis for the alteration of coenzyme specificity in a malate dehydrogenase mutant
|
|
21504
|
Structural basis for the alteration of coenzyme specificity in a malate dehydrogenase mutant
|
|
21505
|
Structural basis for the alteration of coenzyme specificity in a malate dehydrogenase mutant
|
|
21506
|
Structural basis for the alteration of coenzyme specificity in a malate dehydrogenase mutant
|
|
21507
|
Purification, characterization and molecular cloning of human hepatic lysosomal acid lipase
|
|
21508
|
Purification, characterization and molecular cloning of human hepatic lysosomal acid lipase
|
|
21509
|
Purification and properties of an NADP + -dependent glycerol dehydrogenase from rabbit skeletal muscle
|
|
21510
|
Purification and properties of an NADP + -dependent glycerol dehydrogenase from rabbit skeletal muscle
|
|
21511
|
Spectral and catalytic properties of aryl-alcohol oxidase, a fungal flavoenzyme acting on polyunsaturated ...
|
|
21512
|
Spectral and catalytic properties of aryl-alcohol oxidase, a fungal flavoenzyme acting on polyunsaturated ...
|
|
21513
|
Spectral and catalytic properties of aryl-alcohol oxidase, a fungal flavoenzyme acting on polyunsaturated ...
|
|
21514
|
Spectral and catalytic properties of aryl-alcohol oxidase, a fungal flavoenzyme acting on polyunsaturated ...
|
|
21515
|
Spectral and catalytic properties of aryl-alcohol oxidase, a fungal flavoenzyme acting on polyunsaturated ...
|
|
21516
|
Spectral and catalytic properties of aryl-alcohol oxidase, a fungal flavoenzyme acting on polyunsaturated ...
|
|
21517
|
Spectral and catalytic properties of aryl-alcohol oxidase, a fungal flavoenzyme acting on polyunsaturated ...
|
|
21518
|
Spectral and catalytic properties of aryl-alcohol oxidase, a fungal flavoenzyme acting on polyunsaturated ...
|
|
21519
|
Spectral and catalytic properties of aryl-alcohol oxidase, a fungal flavoenzyme acting on polyunsaturated ...
|
|
21520
|
Spectral and catalytic properties of aryl-alcohol oxidase, a fungal flavoenzyme acting on polyunsaturated ...
|
|
21521
|
Spectral and catalytic properties of aryl-alcohol oxidase, a fungal flavoenzyme acting on polyunsaturated ...
|
|
21522
|
Spectral and catalytic properties of aryl-alcohol oxidase, a fungal flavoenzyme acting on polyunsaturated ...
|
|
21523
|
Spectral and catalytic properties of aryl-alcohol oxidase, a fungal flavoenzyme acting on polyunsaturated ...
|
|
21524
|
Spectral and catalytic properties of aryl-alcohol oxidase, a fungal flavoenzyme acting on polyunsaturated ...
|
|
21525
|
Spectral and catalytic properties of aryl-alcohol oxidase, a fungal flavoenzyme acting on polyunsaturated ...
|
|
21526
|
Spectral and catalytic properties of aryl-alcohol oxidase, a fungal flavoenzyme acting on polyunsaturated ...
|
|
21527
|
Spectral and catalytic properties of aryl-alcohol oxidase, a fungal flavoenzyme acting on polyunsaturated ...
|
|
21528
|
Spectral and catalytic properties of aryl-alcohol oxidase, a fungal flavoenzyme acting on polyunsaturated ...
|
|
21529
|
Structure-activity relationships of 3-deoxy androgens as aromatase inhibitors. Synthesis and biochemical ...
|
|
21530
|
Structure-activity relationships of 3-deoxy androgens as aromatase inhibitors. Synthesis and biochemical ...
|
|
21531
|
Structure-activity relationships of 3-deoxy androgens as aromatase inhibitors. Synthesis and biochemical ...
|
|
21532
|
Structure-activity relationships of 3-deoxy androgens as aromatase inhibitors. Synthesis and biochemical ...
|
|
21533
|
Structure-activity relationships of 3-deoxy androgens as aromatase inhibitors. Synthesis and biochemical ...
|
|
21534
|
Structure-activity relationships of 3-deoxy androgens as aromatase inhibitors. Synthesis and biochemical ...
|
|
21535
|
Structure-activity relationships of 3-deoxy androgens as aromatase inhibitors. Synthesis and biochemical ...
|
|
21536
|
Structure-activity relationships of 3-deoxy androgens as aromatase inhibitors. Synthesis and biochemical ...
|
|
21537
|
Structure-activity relationships of 3-deoxy androgens as aromatase inhibitors. Synthesis and biochemical ...
|
|
21538
|
Structure-activity relationships of 3-deoxy androgens as aromatase inhibitors. Synthesis and biochemical ...
|
|
21539
|
Structure-activity relationships of 3-deoxy androgens as aromatase inhibitors. Synthesis and biochemical ...
|
|
21540
|
Structure-activity relationships of 3-deoxy androgens as aromatase inhibitors. Synthesis and biochemical ...
|
|
21541
|
Structure-activity relationships of 3-deoxy androgens as aromatase inhibitors. Synthesis and biochemical ...
|
|
21542
|
Farnesyl-diphosphate synthase is localized in peroxisomes
|
|
21543
|
Farnesyl-diphosphate synthase is localized in peroxisomes
|
|
21544
|
Farnesyl-diphosphate synthase is localized in peroxisomes
|
|
21545
|
Farnesyl-diphosphate synthase is localized in peroxisomes
|
|
21546
|
Farnesyl-diphosphate synthase is localized in peroxisomes
|
|
21547
|
Farnesyl-diphosphate synthase is localized in peroxisomes
|
|
21548
|
Farnesyl-diphosphate synthase is localized in peroxisomes
|
|
21549
|
Farnesyl-diphosphate synthase is localized in peroxisomes
|
|
21550
|
Farnesyl-diphosphate synthase is localized in peroxisomes
|
|
21551
|
Farnesyl-diphosphate synthase is localized in peroxisomes
|
|
21552
|
Farnesyl-diphosphate synthase is localized in peroxisomes
|
|
21553
|
Farnesyl-diphosphate synthase is localized in peroxisomes
|
|
21554
|
Farnesyl-diphosphate synthase is localized in peroxisomes
|
|
21555
|
Farnesyl-diphosphate synthase is localized in peroxisomes
|
|
21556
|
Farnesyl-diphosphate synthase is localized in peroxisomes
|
|
21557
|
Farnesyl-diphosphate synthase is localized in peroxisomes
|
|
21558
|
Farnesyl-diphosphate synthase is localized in peroxisomes
|
|
21559
|
Farnesyl-diphosphate synthase is localized in peroxisomes
|
|
21560
|
Purification and regulatory properties of pigeon erythrocyte pyruvate kinase.
|
|
21561
|
Purification and regulatory properties of pigeon erythrocyte pyruvate kinase.
|
|
21562
|
Purification and regulatory properties of pigeon erythrocyte pyruvate kinase.
|
|
21563
|
Purification and regulatory properties of pigeon erythrocyte pyruvate kinase.
|
|
21564
|
Two additional glutaredoxins exist in Escherichia coli: glutaredoxin 3 is a hydrogen donor for ribonucleotide ...
|
|
21565
|
Two additional glutaredoxins exist in Escherichia coli: glutaredoxin 3 is a hydrogen donor for ribonucleotide ...
|
|
21566
|
THE ENZYMES OF THE GALACTOSE OPERON IN ESCHERICHIA COLI. I. PURIFICATION AND CHARACTERIZATION OF URIDINE ...
|
|
21567
|
Purification and properties of the manganese-dependent phosphoglycerate mutase of Bacillus subtilis
|
|
21568
|
Purification and properties of the manganese-dependent phosphoglycerate mutase of Bacillus subtilis
|
|
21569
|
Purification and properties of the manganese-dependent phosphoglycerate mutase of Bacillus subtilis
|
|
21570
|
Purification and properties of the manganese-dependent phosphoglycerate mutase of Bacillus subtilis
|
|
21571
|
Modulation of the conformation of a membrane glycosyltransferase by specific lipids
|
|
21572
|
Modulation of the conformation of a membrane glycosyltransferase by specific lipids
|
|
21573
|
Cloning, overexpression, and characterization of glutaredoxin 2, an atypical glutaredoxin from Escherichia ...
|
|
21574
|
Cloning, overexpression, and characterization of glutaredoxin 2, an atypical glutaredoxin from Escherichia ...
|
|
21575
|
Cloning, overexpression, and characterization of glutaredoxin 2, an atypical glutaredoxin from Escherichia ...
|
|
21576
|
Cloning, overexpression, and characterization of glutaredoxin 2, an atypical glutaredoxin from Escherichia ...
|
|
21577
|
Alteration of the quaternary structure of human UDP-glucose dehydrogenase by a double mutation
|
|
21578
|
Alteration of the quaternary structure of human UDP-glucose dehydrogenase by a double mutation
|
|
21579
|
Alteration of the quaternary structure of human UDP-glucose dehydrogenase by a double mutation
|
|
21580
|
Alteration of the quaternary structure of human UDP-glucose dehydrogenase by a double mutation
|
|
21581
|
Conversion of a glutamate dehydrogenase into methionine/norleucine dehydrogenase by site-directed mutagenesis
|
|
21582
|
Conversion of a glutamate dehydrogenase into methionine/norleucine dehydrogenase by site-directed mutagenesis
|
|
21583
|
Conversion of a glutamate dehydrogenase into methionine/norleucine dehydrogenase by site-directed mutagenesis
|
|
21584
|
Conversion of a glutamate dehydrogenase into methionine/norleucine dehydrogenase by site-directed mutagenesis
|
|
21585
|
Conversion of a glutamate dehydrogenase into methionine/norleucine dehydrogenase by site-directed mutagenesis
|
|
21586
|
Conversion of a glutamate dehydrogenase into methionine/norleucine dehydrogenase by site-directed mutagenesis
|
|
21587
|
Conversion of a glutamate dehydrogenase into methionine/norleucine dehydrogenase by site-directed mutagenesis
|
|
21588
|
Conversion of a glutamate dehydrogenase into methionine/norleucine dehydrogenase by site-directed mutagenesis
|
|
21589
|
Conversion of a glutamate dehydrogenase into methionine/norleucine dehydrogenase by site-directed mutagenesis
|
|
21590
|
Conversion of a glutamate dehydrogenase into methionine/norleucine dehydrogenase by site-directed mutagenesis
|
|
21591
|
Conversion of a glutamate dehydrogenase into methionine/norleucine dehydrogenase by site-directed mutagenesis
|
|
21592
|
Conversion of a glutamate dehydrogenase into methionine/norleucine dehydrogenase by site-directed mutagenesis
|
|
21593
|
Conversion of a glutamate dehydrogenase into methionine/norleucine dehydrogenase by site-directed mutagenesis
|
|
21594
|
Conversion of a glutamate dehydrogenase into methionine/norleucine dehydrogenase by site-directed mutagenesis
|
|
21595
|
Conversion of a glutamate dehydrogenase into methionine/norleucine dehydrogenase by site-directed mutagenesis
|
|
21596
|
Conversion of a glutamate dehydrogenase into methionine/norleucine dehydrogenase by site-directed mutagenesis
|
|
21597
|
Conversion of a glutamate dehydrogenase into methionine/norleucine dehydrogenase by site-directed mutagenesis
|
|
21598
|
Conversion of a glutamate dehydrogenase into methionine/norleucine dehydrogenase by site-directed mutagenesis
|
|
21599
|
Conversion of a glutamate dehydrogenase into methionine/norleucine dehydrogenase by site-directed mutagenesis
|
|
21600
|
Conversion of a glutamate dehydrogenase into methionine/norleucine dehydrogenase by site-directed mutagenesis
|
|
21601
|
Conversion of a glutamate dehydrogenase into methionine/norleucine dehydrogenase by site-directed mutagenesis
|
|
21602
|
Conversion of a glutamate dehydrogenase into methionine/norleucine dehydrogenase by site-directed mutagenesis
|
|
21603
|
Conversion of a glutamate dehydrogenase into methionine/norleucine dehydrogenase by site-directed mutagenesis
|
|
21604
|
Conversion of a glutamate dehydrogenase into methionine/norleucine dehydrogenase by site-directed mutagenesis
|
|
21605
|
Conversion of a glutamate dehydrogenase into methionine/norleucine dehydrogenase by site-directed mutagenesis
|
|
21606
|
Conversion of a glutamate dehydrogenase into methionine/norleucine dehydrogenase by site-directed mutagenesis
|
|
21607
|
Conversion of a glutamate dehydrogenase into methionine/norleucine dehydrogenase by site-directed mutagenesis
|
|
21608
|
Conversion of a glutamate dehydrogenase into methionine/norleucine dehydrogenase by site-directed mutagenesis
|
|
21609
|
Conversion of a glutamate dehydrogenase into methionine/norleucine dehydrogenase by site-directed mutagenesis
|
|
21610
|
Conversion of a glutamate dehydrogenase into methionine/norleucine dehydrogenase by site-directed mutagenesis
|
|
21611
|
Conversion of a glutamate dehydrogenase into methionine/norleucine dehydrogenase by site-directed mutagenesis
|
|
21612
|
Conversion of a glutamate dehydrogenase into methionine/norleucine dehydrogenase by site-directed mutagenesis
|
|
21613
|
Conversion of a glutamate dehydrogenase into methionine/norleucine dehydrogenase by site-directed mutagenesis
|
|
21614
|
Conversion of a glutamate dehydrogenase into methionine/norleucine dehydrogenase by site-directed mutagenesis
|
|
21615
|
Conversion of a glutamate dehydrogenase into methionine/norleucine dehydrogenase by site-directed mutagenesis
|
|
21616
|
Conversion of a glutamate dehydrogenase into methionine/norleucine dehydrogenase by site-directed mutagenesis
|
|
21617
|
Conversion of a glutamate dehydrogenase into methionine/norleucine dehydrogenase by site-directed mutagenesis
|
|
21618
|
Conversion of a glutamate dehydrogenase into methionine/norleucine dehydrogenase by site-directed mutagenesis
|
|
21619
|
Conversion of a glutamate dehydrogenase into methionine/norleucine dehydrogenase by site-directed mutagenesis
|
|
21620
|
Characteristics and crystal structure of bacterial inosine-5`-monophosphate dehydrogenase
|
|
21621
|
Characteristics and crystal structure of bacterial inosine-5`-monophosphate dehydrogenase
|
|
21622
|
Characteristics and crystal structure of bacterial inosine-5`-monophosphate dehydrogenase
|
|
21623
|
Characteristics and crystal structure of bacterial inosine-5`-monophosphate dehydrogenase
|
|
21624
|
Characteristics and crystal structure of bacterial inosine-5`-monophosphate dehydrogenase
|
|
21625
|
A bifunctional enzyme catalyzes the first two steps in N-acetylneuraminic acid biosynthesis of rat liver. ...
|
|
21626
|
A bifunctional enzyme catalyzes the first two steps in N-acetylneuraminic acid biosynthesis of rat liver. ...
|
|
21627
|
A bifunctional enzyme catalyzes the first two steps in N-acetylneuraminic acid biosynthesis of rat liver. ...
|
|
21628
|
A bifunctional enzyme catalyzes the first two steps in N-acetylneuraminic acid biosynthesis of rat liver. ...
|
|
21629
|
A bifunctional enzyme catalyzes the first two steps in N-acetylneuraminic acid biosynthesis of rat liver. ...
|
|
21630
|
A bifunctional enzyme catalyzes the first two steps in N-acetylneuraminic acid biosynthesis of rat liver. ...
|
|
21631
|
Dramatic broadening of the substrate profile of the Aeromonas hydrophila CphA metallo-beta-lactamase by ...
|
|
21632
|
Dramatic broadening of the substrate profile of the Aeromonas hydrophila CphA metallo-beta-lactamase by ...
|
|
21633
|
Dramatic broadening of the substrate profile of the Aeromonas hydrophila CphA metallo-beta-lactamase by ...
|
|
21634
|
Dramatic broadening of the substrate profile of the Aeromonas hydrophila CphA metallo-beta-lactamase by ...
|
|
21635
|
Dramatic broadening of the substrate profile of the Aeromonas hydrophila CphA metallo-beta-lactamase by ...
|
|
21636
|
Dramatic broadening of the substrate profile of the Aeromonas hydrophila CphA metallo-beta-lactamase by ...
|
|
21637
|
Dramatic broadening of the substrate profile of the Aeromonas hydrophila CphA metallo-beta-lactamase by ...
|
|
21638
|
Dramatic broadening of the substrate profile of the Aeromonas hydrophila CphA metallo-beta-lactamase by ...
|
|
21639
|
Dramatic broadening of the substrate profile of the Aeromonas hydrophila CphA metallo-beta-lactamase by ...
|
|
21640
|
Dramatic broadening of the substrate profile of the Aeromonas hydrophila CphA metallo-beta-lactamase by ...
|
|
21641
|
Dramatic broadening of the substrate profile of the Aeromonas hydrophila CphA metallo-beta-lactamase by ...
|
|
21642
|
Dramatic broadening of the substrate profile of the Aeromonas hydrophila CphA metallo-beta-lactamase by ...
|
|
21643
|
Dramatic broadening of the substrate profile of the Aeromonas hydrophila CphA metallo-beta-lactamase by ...
|
|
21644
|
Dramatic broadening of the substrate profile of the Aeromonas hydrophila CphA metallo-beta-lactamase by ...
|
|
21645
|
Dramatic broadening of the substrate profile of the Aeromonas hydrophila CphA metallo-beta-lactamase by ...
|
|
21646
|
Dramatic broadening of the substrate profile of the Aeromonas hydrophila CphA metallo-beta-lactamase by ...
|
|
21647
|
Dramatic broadening of the substrate profile of the Aeromonas hydrophila CphA metallo-beta-lactamase by ...
|
|
21648
|
Dramatic broadening of the substrate profile of the Aeromonas hydrophila CphA metallo-beta-lactamase by ...
|
|
21649
|
Dramatic broadening of the substrate profile of the Aeromonas hydrophila CphA metallo-beta-lactamase by ...
|
|
21650
|
Dramatic broadening of the substrate profile of the Aeromonas hydrophila CphA metallo-beta-lactamase by ...
|
|
21651
|
Dramatic broadening of the substrate profile of the Aeromonas hydrophila CphA metallo-beta-lactamase by ...
|
|
21652
|
Dramatic broadening of the substrate profile of the Aeromonas hydrophila CphA metallo-beta-lactamase by ...
|
|
21653
|
Dramatic broadening of the substrate profile of the Aeromonas hydrophila CphA metallo-beta-lactamase by ...
|
|
21654
|
Dramatic broadening of the substrate profile of the Aeromonas hydrophila CphA metallo-beta-lactamase by ...
|
|
21655
|
Dramatic broadening of the substrate profile of the Aeromonas hydrophila CphA metallo-beta-lactamase by ...
|
|
21656
|
Dramatic broadening of the substrate profile of the Aeromonas hydrophila CphA metallo-beta-lactamase by ...
|
|
21657
|
Dramatic broadening of the substrate profile of the Aeromonas hydrophila CphA metallo-beta-lactamase by ...
|
|
21658
|
Dramatic broadening of the substrate profile of the Aeromonas hydrophila CphA metallo-beta-lactamase by ...
|
|
21659
|
Dramatic broadening of the substrate profile of the Aeromonas hydrophila CphA metallo-beta-lactamase by ...
|
|
21660
|
Dramatic broadening of the substrate profile of the Aeromonas hydrophila CphA metallo-beta-lactamase by ...
|
|
21661
|
Dramatic broadening of the substrate profile of the Aeromonas hydrophila CphA metallo-beta-lactamase by ...
|
|
21662
|
Dramatic broadening of the substrate profile of the Aeromonas hydrophila CphA metallo-beta-lactamase by ...
|
|
21663
|
Dramatic broadening of the substrate profile of the Aeromonas hydrophila CphA metallo-beta-lactamase by ...
|
|
21664
|
Dramatic broadening of the substrate profile of the Aeromonas hydrophila CphA metallo-beta-lactamase by ...
|
|
21665
|
Dramatic broadening of the substrate profile of the Aeromonas hydrophila CphA metallo-beta-lactamase by ...
|
|
21666
|
Dramatic broadening of the substrate profile of the Aeromonas hydrophila CphA metallo-beta-lactamase by ...
|
|
21667
|
Dramatic broadening of the substrate profile of the Aeromonas hydrophila CphA metallo-beta-lactamase by ...
|
|
21668
|
Membrane-bound DD-carboxypeptidase and LD-transpeptidase of Streptococcus faecalis ATCC 9790
|
|
21669
|
Membrane-bound DD-carboxypeptidase and LD-transpeptidase of Streptococcus faecalis ATCC 9790
|
|
21670
|
Membrane-bound DD-carboxypeptidase and LD-transpeptidase of Streptococcus faecalis ATCC 9790
|
|
21671
|
Comparison of skin esterase activities from different species
|
|
21672
|
Comparison of skin esterase activities from different species
|
|
21673
|
Comparison of skin esterase activities from different species
|
|
21674
|
Comparison of skin esterase activities from different species
|
|
21675
|
Comparison of skin esterase activities from different species
|
|
21676
|
Comparison of skin esterase activities from different species
|
|
21677
|
Comparison of skin esterase activities from different species
|
|
21678
|
Comparison of skin esterase activities from different species
|
|
21679
|
Comparison of skin esterase activities from different species
|
|
21680
|
Comparison of skin esterase activities from different species
|
|
21681
|
Comparison of skin esterase activities from different species
|
|
21682
|
Comparison of skin esterase activities from different species
|
|
21683
|
Comparison of skin esterase activities from different species
|
|
21684
|
Comparison of skin esterase activities from different species
|
|
21685
|
Comparison of skin esterase activities from different species
|
|
21686
|
Comparison of skin esterase activities from different species
|
|
21687
|
Comparison of skin esterase activities from different species
|
|
21688
|
Comparison of skin esterase activities from different species
|
|
21689
|
Comparison of skin esterase activities from different species
|
|
21690
|
Comparison of skin esterase activities from different species
|
|
21691
|
Comparison of skin esterase activities from different species
|
|
21692
|
Comparison of skin esterase activities from different species
|
|
21693
|
Comparison of skin esterase activities from different species
|
|
21694
|
Comparison of skin esterase activities from different species
|
|
21695
|
Comparison of skin esterase activities from different species
|
|
21696
|
Comparison of skin esterase activities from different species
|
|
21697
|
Comparison of skin esterase activities from different species
|
|
21698
|
Comparison of skin esterase activities from different species
|
|
21699
|
Comparison of skin esterase activities from different species
|
|
21700
|
Comparison of skin esterase activities from different species
|
|
21701
|
Comparison of skin esterase activities from different species
|
|
21702
|
Comparison of skin esterase activities from different species
|
|
21703
|
Comparison of skin esterase activities from different species
|
|
21704
|
Comparison of skin esterase activities from different species
|
|
21705
|
Comparison of skin esterase activities from different species
|
|
21706
|
Comparison of skin esterase activities from different species
|
|
21707
|
Comparison of skin esterase activities from different species
|
|
21708
|
Comparison of skin esterase activities from different species
|
|
21709
|
Comparison of skin esterase activities from different species
|
|
21710
|
Comparison of skin esterase activities from different species
|
|
21711
|
Structural basis for glutamate racemase inhibition
|
|
21712
|
Structural basis for glutamate racemase inhibition
|
|
21713
|
Importance of Gly-13 for the Coenzyme Binding of Human UDP-glucose Dehydrogenase
|
|
21714
|
Importance of Gly-13 for the Coenzyme Binding of Human UDP-glucose Dehydrogenase
|
|
21715
|
Crystallization and reaction mechanism of yeast phosphoglucomutase
|
|
21716
|
Molecular cloning and characterization of one member of 3beta-hydroxy sterol glucosyltransferase gene family ...
|
|
21717
|
Molecular cloning and characterization of one member of 3beta-hydroxy sterol glucosyltransferase gene family ...
|
|
21718
|
Molecular cloning and characterization of one member of 3beta-hydroxy sterol glucosyltransferase gene family ...
|
|
21719
|
Molecular cloning and characterization of one member of 3beta-hydroxy sterol glucosyltransferase gene family ...
|
|
21720
|
Molecular cloning and characterization of one member of 3beta-hydroxy sterol glucosyltransferase gene family ...
|
|
21721
|
Molecular cloning and characterization of one member of 3beta-hydroxy sterol glucosyltransferase gene family ...
|
|
21722
|
Molecular cloning and characterization of one member of 3beta-hydroxy sterol glucosyltransferase gene family ...
|
|
21723
|
Molecular cloning and characterization of one member of 3beta-hydroxy sterol glucosyltransferase gene family ...
|
|
21724
|
Molecular cloning and characterization of one member of 3beta-hydroxy sterol glucosyltransferase gene family ...
|
|
21725
|
Molecular cloning and characterization of one member of 3beta-hydroxy sterol glucosyltransferase gene family ...
|
|
21726
|
Molecular cloning and characterization of one member of 3beta-hydroxy sterol glucosyltransferase gene family ...
|
|
21727
|
Molecular cloning and characterization of one member of 3beta-hydroxy sterol glucosyltransferase gene family ...
|
|
21728
|
Molecular cloning and characterization of one member of 3beta-hydroxy sterol glucosyltransferase gene family ...
|
|
21729
|
Molecular cloning and characterization of one member of 3beta-hydroxy sterol glucosyltransferase gene family ...
|
|
21730
|
Molecular cloning and characterization of one member of 3beta-hydroxy sterol glucosyltransferase gene family ...
|
|
21731
|
Molecular cloning and characterization of one member of 3beta-hydroxy sterol glucosyltransferase gene family ...
|
|
21732
|
Molecular cloning and characterization of one member of 3beta-hydroxy sterol glucosyltransferase gene family ...
|
|
21733
|
Molecular cloning and characterization of one member of 3beta-hydroxy sterol glucosyltransferase gene family ...
|
|
21734
|
Molecular cloning and characterization of one member of 3beta-hydroxy sterol glucosyltransferase gene family ...
|
|
21735
|
Molecular cloning and characterization of one member of 3beta-hydroxy sterol glucosyltransferase gene family ...
|
|
21736
|
Molecular cloning and characterization of one member of 3beta-hydroxy sterol glucosyltransferase gene family ...
|
|
21737
|
Molecular cloning and characterization of one member of 3beta-hydroxy sterol glucosyltransferase gene family ...
|
|
21738
|
Molecular cloning and characterization of one member of 3beta-hydroxy sterol glucosyltransferase gene family ...
|
|
21739
|
Molecular cloning and characterization of one member of 3beta-hydroxy sterol glucosyltransferase gene family ...
|
|
21740
|
Properties of extracellular neuraminidase produced by group A streptococcus
|
|
21741
|
Purification and properties of streptococcal hyaluronate lyase
|
|
21742
|
Partial Reversal of the Substrate Stereospecificity of an L-Lactate Dehydrogenase by Site-Directed Mutagenesis
|
|
21743
|
Partial Reversal of the Substrate Stereospecificity of an L-Lactate Dehydrogenase by Site-Directed Mutagenesis
|
|
21744
|
Partial Reversal of the Substrate Stereospecificity of an L-Lactate Dehydrogenase by Site-Directed Mutagenesis
|
|
21745
|
Partial Reversal of the Substrate Stereospecificity of an L-Lactate Dehydrogenase by Site-Directed Mutagenesis
|
|
21746
|
Partial Reversal of the Substrate Stereospecificity of an L-Lactate Dehydrogenase by Site-Directed Mutagenesis
|
|
21747
|
Partial Reversal of the Substrate Stereospecificity of an L-Lactate Dehydrogenase by Site-Directed Mutagenesis
|
|
21748
|
Partial Reversal of the Substrate Stereospecificity of an L-Lactate Dehydrogenase by Site-Directed Mutagenesis
|
|
21749
|
Partial Reversal of the Substrate Stereospecificity of an L-Lactate Dehydrogenase by Site-Directed Mutagenesis
|
|
21750
|
Partial Reversal of the Substrate Stereospecificity of an L-Lactate Dehydrogenase by Site-Directed Mutagenesis
|
|
21751
|
Partial Reversal of the Substrate Stereospecificity of an L-Lactate Dehydrogenase by Site-Directed Mutagenesis
|
|
21752
|
Partial Reversal of the Substrate Stereospecificity of an L-Lactate Dehydrogenase by Site-Directed Mutagenesis
|
|
21753
|
Partial Reversal of the Substrate Stereospecificity of an L-Lactate Dehydrogenase by Site-Directed Mutagenesis
|
|
21754
|
Partial Reversal of the Substrate Stereospecificity of an L-Lactate Dehydrogenase by Site-Directed Mutagenesis
|
|
21755
|
Partial Reversal of the Substrate Stereospecificity of an L-Lactate Dehydrogenase by Site-Directed Mutagenesis
|
|
21756
|
Partial Reversal of the Substrate Stereospecificity of an L-Lactate Dehydrogenase by Site-Directed Mutagenesis
|
|
21757
|
Partial Reversal of the Substrate Stereospecificity of an L-Lactate Dehydrogenase by Site-Directed Mutagenesis
|
|
21758
|
Partial Reversal of the Substrate Stereospecificity of an L-Lactate Dehydrogenase by Site-Directed Mutagenesis
|
|
21759
|
Partial Reversal of the Substrate Stereospecificity of an L-Lactate Dehydrogenase by Site-Directed Mutagenesis
|
|
21760
|
Partial Reversal of the Substrate Stereospecificity of an L-Lactate Dehydrogenase by Site-Directed Mutagenesis
|
|
21761
|
Partial Reversal of the Substrate Stereospecificity of an L-Lactate Dehydrogenase by Site-Directed Mutagenesis
|
|
21762
|
Partial Reversal of the Substrate Stereospecificity of an L-Lactate Dehydrogenase by Site-Directed Mutagenesis
|
|
21763
|
Partial Reversal of the Substrate Stereospecificity of an L-Lactate Dehydrogenase by Site-Directed Mutagenesis
|
|
21764
|
Partial Reversal of the Substrate Stereospecificity of an L-Lactate Dehydrogenase by Site-Directed Mutagenesis
|
|
21765
|
Partial Reversal of the Substrate Stereospecificity of an L-Lactate Dehydrogenase by Site-Directed Mutagenesis
|
|
21766
|
Partial Reversal of the Substrate Stereospecificity of an L-Lactate Dehydrogenase by Site-Directed Mutagenesis
|
|
21767
|
Partial Reversal of the Substrate Stereospecificity of an L-Lactate Dehydrogenase by Site-Directed Mutagenesis
|
|
21768
|
Partial Reversal of the Substrate Stereospecificity of an L-Lactate Dehydrogenase by Site-Directed Mutagenesis
|
|
21769
|
Partial Reversal of the Substrate Stereospecificity of an L-Lactate Dehydrogenase by Site-Directed Mutagenesis
|
|
21770
|
Partial Reversal of the Substrate Stereospecificity of an L-Lactate Dehydrogenase by Site-Directed Mutagenesis
|
|
21771
|
Partial Reversal of the Substrate Stereospecificity of an L-Lactate Dehydrogenase by Site-Directed Mutagenesis
|
|
21772
|
Partial Reversal of the Substrate Stereospecificity of an L-Lactate Dehydrogenase by Site-Directed Mutagenesis
|
|
21773
|
Partial Reversal of the Substrate Stereospecificity of an L-Lactate Dehydrogenase by Site-Directed Mutagenesis
|
|
21774
|
Partial Reversal of the Substrate Stereospecificity of an L-Lactate Dehydrogenase by Site-Directed Mutagenesis
|
|
21775
|
Partial Reversal of the Substrate Stereospecificity of an L-Lactate Dehydrogenase by Site-Directed Mutagenesis
|
|
21776
|
Partial Reversal of the Substrate Stereospecificity of an L-Lactate Dehydrogenase by Site-Directed Mutagenesis
|
|
21777
|
Partial Reversal of the Substrate Stereospecificity of an L-Lactate Dehydrogenase by Site-Directed Mutagenesis
|
|
21778
|
Partial Reversal of the Substrate Stereospecificity of an L-Lactate Dehydrogenase by Site-Directed Mutagenesis
|
|
21779
|
Partial Reversal of the Substrate Stereospecificity of an L-Lactate Dehydrogenase by Site-Directed Mutagenesis
|
|
21780
|
Partial Reversal of the Substrate Stereospecificity of an L-Lactate Dehydrogenase by Site-Directed Mutagenesis
|
|
21781
|
Partial Reversal of the Substrate Stereospecificity of an L-Lactate Dehydrogenase by Site-Directed Mutagenesis
|
|
21782
|
Partial Reversal of the Substrate Stereospecificity of an L-Lactate Dehydrogenase by Site-Directed Mutagenesis
|
|
21783
|
Partial Reversal of the Substrate Stereospecificity of an L-Lactate Dehydrogenase by Site-Directed Mutagenesis
|
|
21784
|
The biosynthesis of cell wall lipopolysaccharide in Escherichia coli. VI. Enzymatic transfer of galactose, ...
|
|
21785
|
The biosynthesis of cell wall lipopolysaccharide in Escherichia coli. VI. Enzymatic transfer of galactose, ...
|
|
21786
|
The biosynthesis of cell wall lipopolysaccharide in Escherichia coli. VI. Enzymatic transfer of galactose, ...
|
|
21787
|
Studies of a phospholipid-requiring bacterial enzyme. I. Purification and properties of uridine diphosphate ...
|
|
21788
|
Hydrolysis of flavin-adenine dinucleotide by rat liver lysosomes
|
|
21789
|
Purification and partial characterization of 3-hydroxyisobutyryl-coenzyme A hydrolase of rat liver
|
|
21790
|
Purification and partial characterization of 3-hydroxyisobutyryl-coenzyme A hydrolase of rat liver
|
|
21791
|
Purification and partial characterization of 3-hydroxyisobutyryl-coenzyme A hydrolase of rat liver
|
|
21792
|
Purification and partial characterization of 3-hydroxyisobutyryl-coenzyme A hydrolase of rat liver
|
|
21793
|
Purification and partial characterization of 3-hydroxyisobutyryl-coenzyme A hydrolase of rat liver
|
|
21794
|
Purification and partial characterization of 3-hydroxyisobutyryl-coenzyme A hydrolase of rat liver
|
|
21795
|
Purification and partial characterization of 3-hydroxyisobutyryl-coenzyme A hydrolase of rat liver
|
|
21796
|
Purification and partial characterization of 3-hydroxyisobutyryl-coenzyme A hydrolase of rat liver
|
|
21797
|
Purification and partial characterization of 3-hydroxyisobutyryl-coenzyme A hydrolase of rat liver
|
|
21798
|
Purification and partial characterization of 3-hydroxyisobutyryl-coenzyme A hydrolase of rat liver
|
|
21799
|
Purification and substrate specificity of a strongly hydrophobic extracellular metalloendopeptidase ...
|
|
21800
|
Purification and substrate specificity of a strongly hydrophobic extracellular metalloendopeptidase ...
|
|
21801
|
Purification and substrate specificity of a strongly hydrophobic extracellular metalloendopeptidase ...
|
|
21802
|
Purification and substrate specificity of a strongly hydrophobic extracellular metalloendopeptidase ...
|
|
21803
|
Citrobacter diversus ULA-27 beta-lactamases. Improved purification and general properties
|
|
21804
|
Citrobacter diversus ULA-27 beta-lactamases. Improved purification and general properties
|
|
21805
|
Citrobacter diversus ULA-27 beta-lactamases. Improved purification and general properties
|
|
21806
|
Citrobacter diversus ULA-27 beta-lactamases. Improved purification and general properties
|
|
21807
|
Citrobacter diversus ULA-27 beta-lactamases. Improved purification and general properties
|
|
21808
|
Citrobacter diversus ULA-27 beta-lactamases. Improved purification and general properties
|
|
21809
|
Citrobacter diversus ULA-27 beta-lactamases. Improved purification and general properties
|
|
21810
|
Citrobacter diversus ULA-27 beta-lactamases. Improved purification and general properties
|
|
21811
|
Citrobacter diversus ULA-27 beta-lactamases. Improved purification and general properties
|
|
21812
|
Citrobacter diversus ULA-27 beta-lactamases. Improved purification and general properties
|
|
21813
|
Citrobacter diversus ULA-27 beta-lactamases. Improved purification and general properties
|
|
21814
|
Citrobacter diversus ULA-27 beta-lactamases. Improved purification and general properties
|
|
21815
|
Citrobacter diversus ULA-27 beta-lactamases. Improved purification and general properties
|
|
21816
|
Citrobacter diversus ULA-27 beta-lactamases. Improved purification and general properties
|
|
21817
|
Citrobacter diversus ULA-27 beta-lactamases. Improved purification and general properties
|
|
21818
|
Citrobacter diversus ULA-27 beta-lactamases. Improved purification and general properties
|
|
21819
|
Citrobacter diversus ULA-27 beta-lactamases. Improved purification and general properties
|
|
21820
|
Citrobacter diversus ULA-27 beta-lactamases. Improved purification and general properties
|
|
21821
|
Citrobacter diversus ULA-27 beta-lactamases. Improved purification and general properties
|
|
21822
|
Citrobacter diversus ULA-27 beta-lactamases. Improved purification and general properties
|
|
21823
|
Citrobacter diversus ULA-27 beta-lactamases. Improved purification and general properties
|
|
21824
|
Citrobacter diversus ULA-27 beta-lactamases. Improved purification and general properties
|
|
21825
|
Citrobacter diversus ULA-27 beta-lactamases. Improved purification and general properties
|
|
21826
|
Citrobacter diversus ULA-27 beta-lactamases. Improved purification and general properties
|
|
21827
|
Citrobacter diversus ULA-27 beta-lactamases. Improved purification and general properties
|
|
21828
|
Citrobacter diversus ULA-27 beta-lactamases. Improved purification and general properties
|
|
21829
|
Citrobacter diversus ULA-27 beta-lactamases. Improved purification and general properties
|
|
21830
|
Citrobacter diversus ULA-27 beta-lactamases. Improved purification and general properties
|
|
21831
|
Overexpression, purification, and characterization of the cloned metallo-beta-lactamase L1 from ...
|
|
21832
|
Overexpression, purification, and characterization of the cloned metallo-beta-lactamase L1 from ...
|
|
21833
|
Overexpression, purification, and characterization of the cloned metallo-beta-lactamase L1 from ...
|
|
21834
|
Overexpression, purification, and characterization of the cloned metallo-beta-lactamase L1 from ...
|
|
21835
|
Overexpression, purification, and characterization of the cloned metallo-beta-lactamase L1 from ...
|
|
21836
|
Overexpression, purification, and characterization of the cloned metallo-beta-lactamase L1 from ...
|
|
21837
|
Overexpression, purification, and characterization of the cloned metallo-beta-lactamase L1 from ...
|
|
21838
|
Overexpression, purification, and characterization of the cloned metallo-beta-lactamase L1 from ...
|
|
21839
|
Overexpression, purification, and characterization of the cloned metallo-beta-lactamase L1 from ...
|
|
21840
|
Overexpression, purification, and characterization of the cloned metallo-beta-lactamase L1 from ...
|
|
21841
|
Overexpression, purification, and characterization of the cloned metallo-beta-lactamase L1 from ...
|
|
21842
|
Overexpression, purification, and characterization of the cloned metallo-beta-lactamase L1 from ...
|
|
21843
|
Overexpression, purification, and characterization of the cloned metallo-beta-lactamase L1 from ...
|
|
21844
|
Overexpression, purification, and characterization of the cloned metallo-beta-lactamase L1 from ...
|
|
21845
|
Overexpression, purification, and characterization of the cloned metallo-beta-lactamase L1 from ...
|
|
21846
|
Overexpression, purification, and characterization of the cloned metallo-beta-lactamase L1 from ...
|
|
21847
|
Overexpression, purification, and characterization of the cloned metallo-beta-lactamase L1 from ...
|
|
21848
|
Overexpression, purification, and characterization of the cloned metallo-beta-lactamase L1 from ...
|
|
21849
|
Overexpression, purification, and characterization of the cloned metallo-beta-lactamase L1 from ...
|
|
21850
|
Overexpression, purification, and characterization of the cloned metallo-beta-lactamase L1 from ...
|
|
21851
|
Overexpression, purification, and characterization of the cloned metallo-beta-lactamase L1 from ...
|
|
21852
|
Overexpression, purification, and characterization of the cloned metallo-beta-lactamase L1 from ...
|
|
21853
|
Overexpression, purification, and characterization of the cloned metallo-beta-lactamase L1 from ...
|
|
21854
|
Overexpression, purification, and characterization of the cloned metallo-beta-lactamase L1 from ...
|
|
21855
|
Overexpression, purification, and characterization of the cloned metallo-beta-lactamase L1 from ...
|
|
21856
|
Overexpression, purification, and characterization of the cloned metallo-beta-lactamase L1 from ...
|
|
21857
|
Overexpression, purification, and characterization of the cloned metallo-beta-lactamase L1 from ...
|
|
21858
|
Overexpression, purification, and characterization of the cloned metallo-beta-lactamase L1 from ...
|
|
21859
|
Overexpression, purification, and characterization of the cloned metallo-beta-lactamase L1 from ...
|
|
21860
|
Overexpression, purification, and characterization of the cloned metallo-beta-lactamase L1 from ...
|
|
21861
|
Isolation and properties of highly purified glutamine transaminase
|
|
21862
|
Isolation and properties of highly purified glutamine transaminase
|
|
21863
|
Isolation and properties of highly purified glutamine transaminase
|
|
21864
|
Isolation and properties of highly purified glutamine transaminase
|
|
21865
|
Isolation and properties of highly purified glutamine transaminase
|
|
21866
|
Isolation and properties of highly purified glutamine transaminase
|
|
21867
|
Isolation and properties of highly purified glutamine transaminase
|
|
21868
|
Isolation and properties of highly purified glutamine transaminase
|
|
21869
|
Isolation and properties of highly purified glutamine transaminase
|
|
21870
|
Isolation and properties of highly purified glutamine transaminase
|
|
21871
|
ATP-sensitive and ATP-insensitive phosphofructokinase in Escherichia coli K-12
|
|
21872
|
ATP-sensitive and ATP-insensitive phosphofructokinase in Escherichia coli K-12
|
|
21873
|
ATP-sensitive and ATP-insensitive phosphofructokinase in Escherichia coli K-12
|
|
21874
|
ATP-sensitive and ATP-insensitive phosphofructokinase in Escherichia coli K-12
|
|
21875
|
ATP-sensitive and ATP-insensitive phosphofructokinase in Escherichia coli K-12
|
|
21876
|
Properties of malonyl-CoA decarboxylase and its relation with malonyl-CoA incorporation into fatty acids by ...
|
|
21877
|
Properties of malonyl-CoA decarboxylase and its relation with malonyl-CoA incorporation into fatty acids by ...
|
|
21878
|
Properties of malonyl-CoA decarboxylase and its relation with malonyl-CoA incorporation into fatty acids by ...
|
|
21879
|
Properties of malonyl-CoA decarboxylase and its relation with malonyl-CoA incorporation into fatty acids by ...
|
|
21880
|
Properties of malonyl-CoA decarboxylase and its relation with malonyl-CoA incorporation into fatty acids by ...
|
|
21881
|
Properties of malonyl-CoA decarboxylase and its relation with malonyl-CoA incorporation into fatty acids by ...
|
|
21882
|
Properties of malonyl-CoA decarboxylase and its relation with malonyl-CoA incorporation into fatty acids by ...
|
|
21883
|
Properties of malonyl-CoA decarboxylase and its relation with malonyl-CoA incorporation into fatty acids by ...
|
|
21884
|
Properties of malonyl-CoA decarboxylase and its relation with malonyl-CoA incorporation into fatty acids by ...
|
|
21885
|
Properties of malonyl-CoA decarboxylase and its relation with malonyl-CoA incorporation into fatty acids by ...
|
|
21886
|
Properties of malonyl-CoA decarboxylase and its relation with malonyl-CoA incorporation into fatty acids by ...
|
|
21887
|
Properties of malonyl-CoA decarboxylase and its relation with malonyl-CoA incorporation into fatty acids by ...
|
|
21888
|
Properties of malonyl-CoA decarboxylase and its relation with malonyl-CoA incorporation into fatty acids by ...
|
|
21889
|
Properties of malonyl-CoA decarboxylase and its relation with malonyl-CoA incorporation into fatty acids by ...
|
|
21890
|
Properties of malonyl-CoA decarboxylase and its relation with malonyl-CoA incorporation into fatty acids by ...
|
|
21891
|
Properties of malonyl-CoA decarboxylase and its relation with malonyl-CoA incorporation into fatty acids by ...
|
|
21892
|
Properties of malonyl-CoA decarboxylase and its relation with malonyl-CoA incorporation into fatty acids by ...
|
|
21893
|
Properties of malonyl-CoA decarboxylase and its relation with malonyl-CoA incorporation into fatty acids by ...
|
|
21894
|
Expression and enzymatic characterization of human glucose phosphate isomerase (GPI) variants accounting for ...
|
|
21895
|
Expression and enzymatic characterization of human glucose phosphate isomerase (GPI) variants accounting for ...
|
|
21896
|
Expression and enzymatic characterization of human glucose phosphate isomerase (GPI) variants accounting for ...
|
|
21897
|
Expression and enzymatic characterization of human glucose phosphate isomerase (GPI) variants accounting for ...
|
|
21898
|
Expression and enzymatic characterization of human glucose phosphate isomerase (GPI) variants accounting for ...
|
|
21899
|
Expression and enzymatic characterization of human glucose phosphate isomerase (GPI) variants accounting for ...
|
|
21900
|
Expression and enzymatic characterization of human glucose phosphate isomerase (GPI) variants accounting for ...
|
|
21901
|
Expression and enzymatic characterization of human glucose phosphate isomerase (GPI) variants accounting for ...
|
|
21902
|
Expression and enzymatic characterization of human glucose phosphate isomerase (GPI) variants accounting for ...
|
|
21903
|
Expression and enzymatic characterization of human glucose phosphate isomerase (GPI) variants accounting for ...
|
|
21904
|
Human liver aldehyde dehydrogenases: new method of purification of the major mitochondrial and cytosolic ...
|
|
21905
|
Human liver aldehyde dehydrogenases: new method of purification of the major mitochondrial and cytosolic ...
|
|
21906
|
Human liver aldehyde dehydrogenases: new method of purification of the major mitochondrial and cytosolic ...
|
|
21907
|
Human liver aldehyde dehydrogenases: new method of purification of the major mitochondrial and cytosolic ...
|
|
21908
|
Human liver aldehyde dehydrogenases: new method of purification of the major mitochondrial and cytosolic ...
|
|
21909
|
Human liver aldehyde dehydrogenases: new method of purification of the major mitochondrial and cytosolic ...
|
|
21910
|
Human liver aldehyde dehydrogenases: new method of purification of the major mitochondrial and cytosolic ...
|
|
21911
|
Human liver aldehyde dehydrogenases: new method of purification of the major mitochondrial and cytosolic ...
|
|
21912
|
Human liver aldehyde dehydrogenases: new method of purification of the major mitochondrial and cytosolic ...
|
|
21913
|
Human liver aldehyde dehydrogenases: new method of purification of the major mitochondrial and cytosolic ...
|
|
21914
|
Kinetic analysis of an inhibitor-resistant variant of the OHIO-1 beta-lactamase, an SHV-family class A enzyme
|
|
21915
|
Kinetic analysis of an inhibitor-resistant variant of the OHIO-1 beta-lactamase, an SHV-family class A enzyme
|
|
21916
|
Kinetic analysis of an inhibitor-resistant variant of the OHIO-1 beta-lactamase, an SHV-family class A enzyme
|
|
21917
|
Kinetic analysis of an inhibitor-resistant variant of the OHIO-1 beta-lactamase, an SHV-family class A enzyme
|
|
21918
|
Kinetic analysis of an inhibitor-resistant variant of the OHIO-1 beta-lactamase, an SHV-family class A enzyme
|
|
21919
|
Kinetic analysis of an inhibitor-resistant variant of the OHIO-1 beta-lactamase, an SHV-family class A enzyme
|
|
21920
|
Kinetic analysis of an inhibitor-resistant variant of the OHIO-1 beta-lactamase, an SHV-family class A enzyme
|
|
21921
|
Kinetic analysis of an inhibitor-resistant variant of the OHIO-1 beta-lactamase, an SHV-family class A enzyme
|
|
21922
|
Kinetic analysis of an inhibitor-resistant variant of the OHIO-1 beta-lactamase, an SHV-family class A enzyme
|
|
21923
|
Kinetic analysis of an inhibitor-resistant variant of the OHIO-1 beta-lactamase, an SHV-family class A enzyme
|
|
21924
|
Kinetic analysis of an inhibitor-resistant variant of the OHIO-1 beta-lactamase, an SHV-family class A enzyme
|
|
21925
|
Kinetic analysis of an inhibitor-resistant variant of the OHIO-1 beta-lactamase, an SHV-family class A enzyme
|
|
21926
|
Kinetic analysis of an inhibitor-resistant variant of the OHIO-1 beta-lactamase, an SHV-family class A enzyme
|
|
21927
|
Kinetic analysis of an inhibitor-resistant variant of the OHIO-1 beta-lactamase, an SHV-family class A enzyme
|
|
21928
|
Kinetic analysis of an inhibitor-resistant variant of the OHIO-1 beta-lactamase, an SHV-family class A enzyme
|
|
21929
|
Kinetic analysis of an inhibitor-resistant variant of the OHIO-1 beta-lactamase, an SHV-family class A enzyme
|
|
21930
|
Disequilibrium in the triose phosphate isomerase system in rat liver
|
|
21931
|
Disequilibrium in the triose phosphate isomerase system in rat liver
|
|
21932
|
Disequilibrium in the triose phosphate isomerase system in rat liver
|
|
21933
|
Disequilibrium in the triose phosphate isomerase system in rat liver
|
|
21934
|
Disequilibrium in the triose phosphate isomerase system in rat liver
|
|
21935
|
Disequilibrium in the triose phosphate isomerase system in rat liver
|
|
21936
|
Identification, purification and characterization of an acetoacetyl-CoA thiolase from rat liver peroxisomes
|
|
21937
|
Identification, purification and characterization of an acetoacetyl-CoA thiolase from rat liver peroxisomes
|
|
21938
|
Identification, purification and characterization of an acetoacetyl-CoA thiolase from rat liver peroxisomes
|
|
21939
|
Identification, purification and characterization of an acetoacetyl-CoA thiolase from rat liver peroxisomes
|
|
21940
|
Identification, purification and characterization of an acetoacetyl-CoA thiolase from rat liver peroxisomes
|
|
21941
|
Identification, purification and characterization of an acetoacetyl-CoA thiolase from rat liver peroxisomes
|
|
21942
|
The use of fluoro- and deoxy-substrate analogs to examine binding specificity and catalysis in the enzymes of ...
|
|
21943
|
The use of fluoro- and deoxy-substrate analogs to examine binding specificity and catalysis in the enzymes of ...
|
|
21944
|
The use of fluoro- and deoxy-substrate analogs to examine binding specificity and catalysis in the enzymes of ...
|
|
21945
|
The use of fluoro- and deoxy-substrate analogs to examine binding specificity and catalysis in the enzymes of ...
|
|
21946
|
The use of fluoro- and deoxy-substrate analogs to examine binding specificity and catalysis in the enzymes of ...
|
|
21947
|
The use of fluoro- and deoxy-substrate analogs to examine binding specificity and catalysis in the enzymes of ...
|
|
21948
|
The use of fluoro- and deoxy-substrate analogs to examine binding specificity and catalysis in the enzymes of ...
|
|
21949
|
The use of fluoro- and deoxy-substrate analogs to examine binding specificity and catalysis in the enzymes of ...
|
|
21950
|
The use of fluoro- and deoxy-substrate analogs to examine binding specificity and catalysis in the enzymes of ...
|
|
21951
|
The use of fluoro- and deoxy-substrate analogs to examine binding specificity and catalysis in the enzymes of ...
|
|
21952
|
The use of fluoro- and deoxy-substrate analogs to examine binding specificity and catalysis in the enzymes of ...
|
|
21953
|
The use of fluoro- and deoxy-substrate analogs to examine binding specificity and catalysis in the enzymes of ...
|
|
21954
|
The use of fluoro- and deoxy-substrate analogs to examine binding specificity and catalysis in the enzymes of ...
|
|
21955
|
The use of fluoro- and deoxy-substrate analogs to examine binding specificity and catalysis in the enzymes of ...
|
|
21956
|
The use of fluoro- and deoxy-substrate analogs to examine binding specificity and catalysis in the enzymes of ...
|
|
21957
|
The use of fluoro- and deoxy-substrate analogs to examine binding specificity and catalysis in the enzymes of ...
|
|
21958
|
The use of fluoro- and deoxy-substrate analogs to examine binding specificity and catalysis in the enzymes of ...
|
|
21959
|
PURIFICATION AND PROPERTIES OF N-ACETYL-D-GLUCOSAMINE KINASE FROM STREPTOCOCCUS PYOGENES
|
|
21960
|
PURIFICATION AND PROPERTIES OF N-ACETYL-D-GLUCOSAMINE KINASE FROM STREPTOCOCCUS PYOGENES
|
|
21961
|
PURIFICATION AND PROPERTIES OF N-ACETYL-D-GLUCOSAMINE KINASE FROM STREPTOCOCCUS PYOGENES
|
|
21962
|
PURIFICATION AND PROPERTIES OF N-ACETYL-D-GLUCOSAMINE KINASE FROM STREPTOCOCCUS PYOGENES
|
|
21963
|
Inducible aldehyde dehydrogenases from rat liver cytosol
|
|
21964
|
Inducible aldehyde dehydrogenases from rat liver cytosol
|
|
21965
|
Inducible aldehyde dehydrogenases from rat liver cytosol
|
|
21966
|
Inducible aldehyde dehydrogenases from rat liver cytosol
|
|
21967
|
Inducible aldehyde dehydrogenases from rat liver cytosol
|
|
21968
|
Inducible aldehyde dehydrogenases from rat liver cytosol
|
|
21969
|
Inducible aldehyde dehydrogenases from rat liver cytosol
|
|
21970
|
Inducible aldehyde dehydrogenases from rat liver cytosol
|
|
21971
|
Inducible aldehyde dehydrogenases from rat liver cytosol
|
|
21972
|
Inducible aldehyde dehydrogenases from rat liver cytosol
|
|
21973
|
Inducible aldehyde dehydrogenases from rat liver cytosol
|
|
21974
|
Inducible aldehyde dehydrogenases from rat liver cytosol
|
|
21975
|
Inducible aldehyde dehydrogenases from rat liver cytosol
|
|
21976
|
Inducible aldehyde dehydrogenases from rat liver cytosol
|
|
21977
|
Inducible aldehyde dehydrogenases from rat liver cytosol
|
|
21978
|
Inducible aldehyde dehydrogenases from rat liver cytosol
|
|
21979
|
Inducible aldehyde dehydrogenases from rat liver cytosol
|
|
21980
|
Inducible aldehyde dehydrogenases from rat liver cytosol
|
|
21981
|
Inducible aldehyde dehydrogenases from rat liver cytosol
|
|
21982
|
Inducible aldehyde dehydrogenases from rat liver cytosol
|
|
21983
|
Inducible aldehyde dehydrogenases from rat liver cytosol
|
|
21984
|
Inducible aldehyde dehydrogenases from rat liver cytosol
|
|
21985
|
Inducible aldehyde dehydrogenases from rat liver cytosol
|
|
21986
|
Inducible aldehyde dehydrogenases from rat liver cytosol
|
|
21987
|
Inducible aldehyde dehydrogenases from rat liver cytosol
|
|
21988
|
Inducible aldehyde dehydrogenases from rat liver cytosol
|
|
21989
|
Inducible aldehyde dehydrogenases from rat liver cytosol
|
|
21990
|
Inducible aldehyde dehydrogenases from rat liver cytosol
|
|
21991
|
Carbamate kinase from Enterococcus faecalis and Enterococcus faecium--cloning of the genes, studies on the ...
|
|
21992
|
Analysis of the kinetic mechanism of the bovine liver mitochondrial dihydroorotate dehydrogenase
|
|
21993
|
Analysis of the kinetic mechanism of the bovine liver mitochondrial dihydroorotate dehydrogenase
|
|
21994
|
Analysis of the kinetic mechanism of the bovine liver mitochondrial dihydroorotate dehydrogenase
|
|
21995
|
Analysis of the kinetic mechanism of the bovine liver mitochondrial dihydroorotate dehydrogenase
|
|
21996
|
Analysis of the kinetic mechanism of the bovine liver mitochondrial dihydroorotate dehydrogenase
|
|
21997
|
Analysis of the kinetic mechanism of the bovine liver mitochondrial dihydroorotate dehydrogenase
|
|
21998
|
Analysis of the kinetic mechanism of the bovine liver mitochondrial dihydroorotate dehydrogenase
|
|
21999
|
Analysis of the kinetic mechanism of the bovine liver mitochondrial dihydroorotate dehydrogenase
|
|
22000
|
Analysis of the kinetic mechanism of the bovine liver mitochondrial dihydroorotate dehydrogenase
|




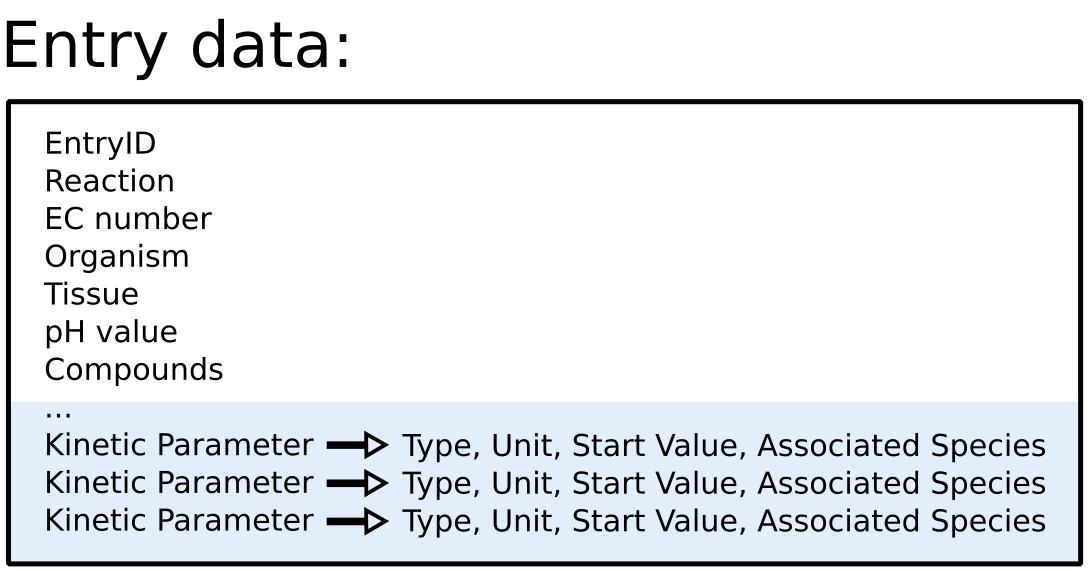
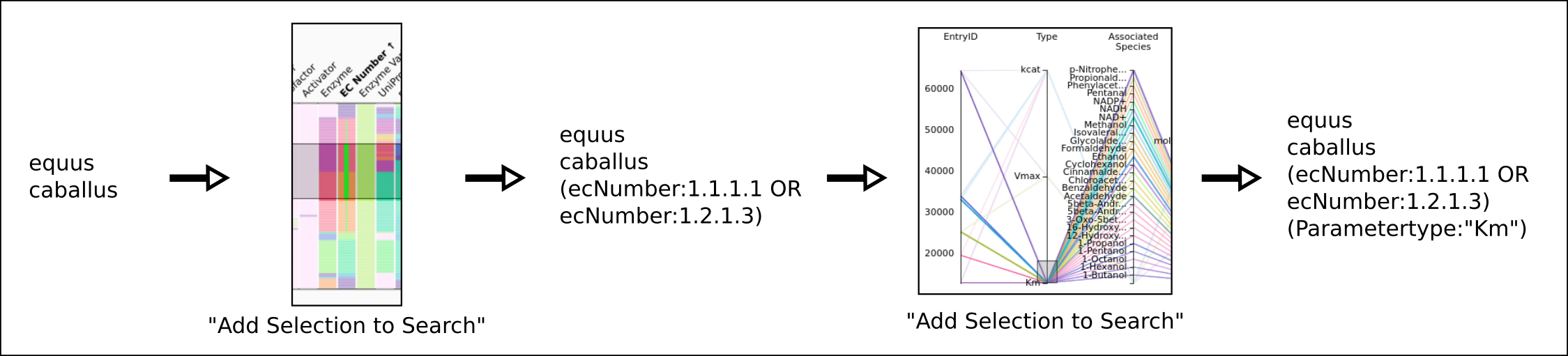 If the current search query is a refinement of the previous query "Use Previous Query (Go Back)" button can be used to perform the previous query again. Only one automatic step back is possible, but the query can be manually manipulated if desired.
If the current search query is a refinement of the previous query "Use Previous Query (Go Back)" button can be used to perform the previous query again. Only one automatic step back is possible, but the query can be manually manipulated if desired.