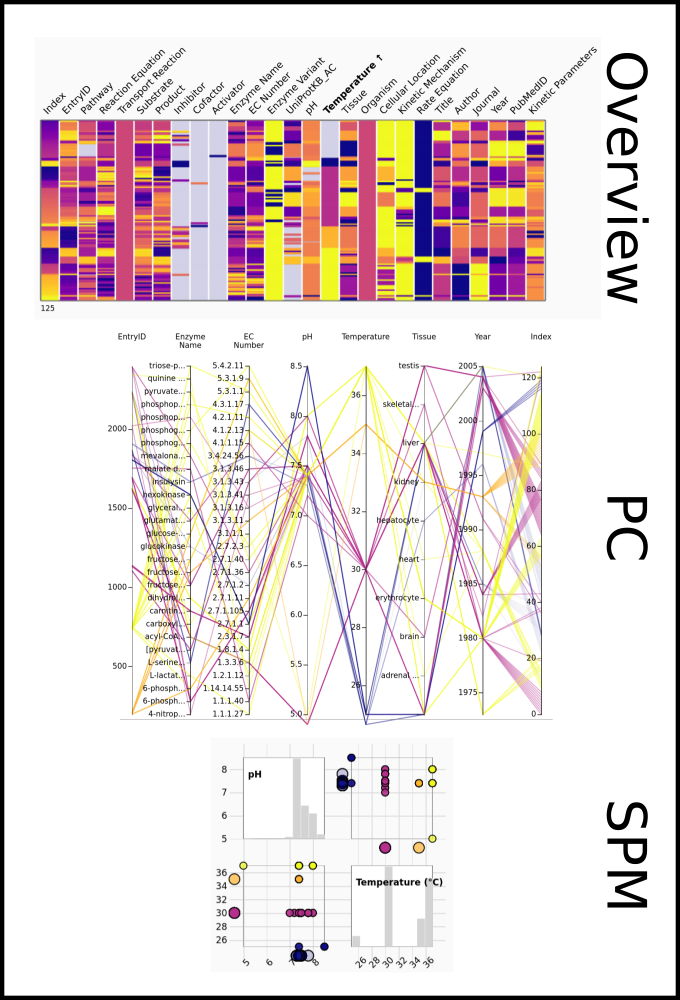
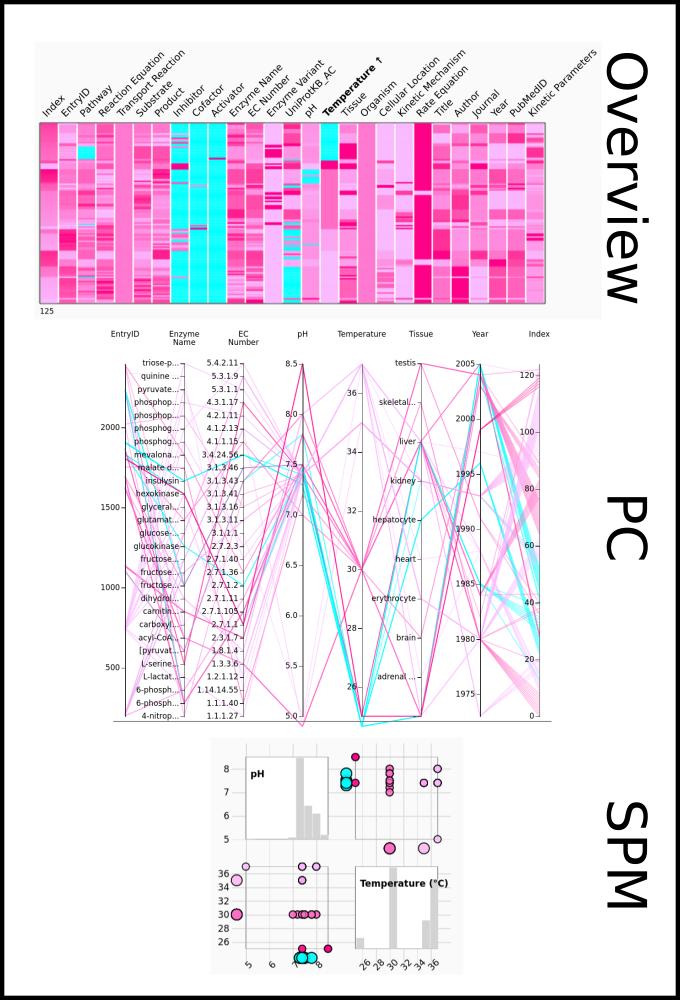
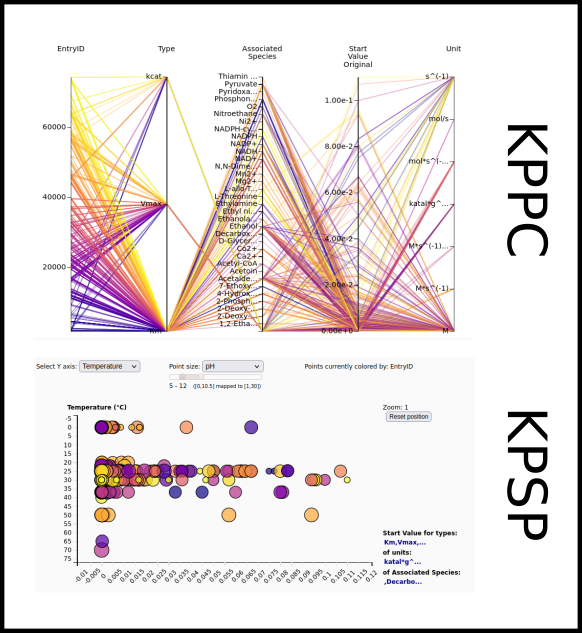
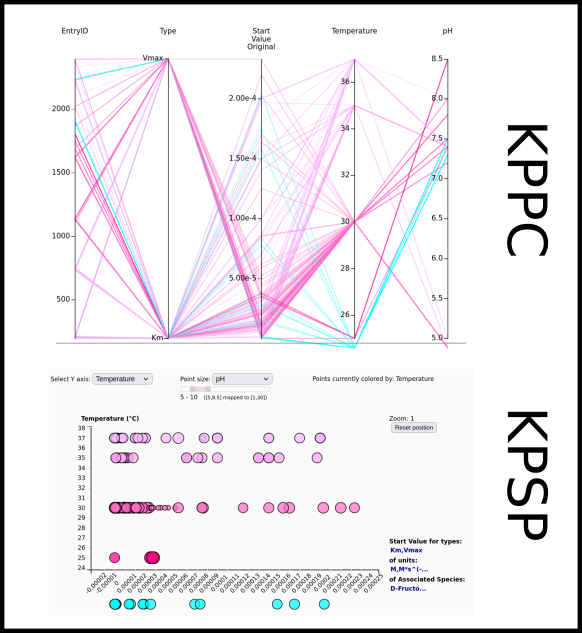
To improve SABIO-RK data interoperability, semantic markup was added to web pages as described and defined by
This structured information makes it easier to discover, collate and analyse our data.
|
41001
|
Purification of mouse liver benzene dihydrodiol dehydrogenases
|
|
41002
|
Purification of mouse liver benzene dihydrodiol dehydrogenases
|
|
41003
|
Purification of mouse liver benzene dihydrodiol dehydrogenases
|
|
41004
|
Purification of mouse liver benzene dihydrodiol dehydrogenases
|
|
41005
|
myo-Inositol-1-Phosphatase from the Pollen of Lilium longiflorum Thunb
|
|
41006
|
myo-Inositol-1-Phosphatase from the Pollen of Lilium longiflorum Thunb
|
|
41007
|
myo-Inositol-1-Phosphatase from the Pollen of Lilium longiflorum Thunb
|
|
41008
|
myo-Inositol-1-Phosphatase from the Pollen of Lilium longiflorum Thunb
|
|
41009
|
myo-Inositol-1-Phosphatase from the Pollen of Lilium longiflorum Thunb
|
|
41010
|
myo-Inositol-1-Phosphatase from the Pollen of Lilium longiflorum Thunb
|
|
41011
|
myo-Inositol-1-Phosphatase from the Pollen of Lilium longiflorum Thunb
|
|
41012
|
myo-Inositol-1-Phosphatase from the Pollen of Lilium longiflorum Thunb
|
|
41013
|
myo-Inositol-1-Phosphatase from the Pollen of Lilium longiflorum Thunb
|
|
41014
|
myo-Inositol-1-Phosphatase from the Pollen of Lilium longiflorum Thunb
|
|
41015
|
Nucleoside ester prodrug substrate specificity of liver carboxylesterase
|
|
41016
|
Nucleoside ester prodrug substrate specificity of liver carboxylesterase
|
|
41017
|
Nucleoside ester prodrug substrate specificity of liver carboxylesterase
|
|
41018
|
Nucleoside ester prodrug substrate specificity of liver carboxylesterase
|
|
41019
|
Nucleoside ester prodrug substrate specificity of liver carboxylesterase
|
|
41020
|
Nucleoside ester prodrug substrate specificity of liver carboxylesterase
|
|
41021
|
Nucleoside ester prodrug substrate specificity of liver carboxylesterase
|
|
41022
|
Nucleoside ester prodrug substrate specificity of liver carboxylesterase
|
|
41023
|
Nucleoside ester prodrug substrate specificity of liver carboxylesterase
|
|
41024
|
Nucleoside ester prodrug substrate specificity of liver carboxylesterase
|
|
41025
|
Nucleoside ester prodrug substrate specificity of liver carboxylesterase
|
|
41026
|
Nucleoside ester prodrug substrate specificity of liver carboxylesterase
|
|
41027
|
Nucleoside ester prodrug substrate specificity of liver carboxylesterase
|
|
41028
|
Nucleoside ester prodrug substrate specificity of liver carboxylesterase
|
|
41029
|
Nucleoside ester prodrug substrate specificity of liver carboxylesterase
|
|
41030
|
Nucleoside ester prodrug substrate specificity of liver carboxylesterase
|
|
41031
|
Nucleoside ester prodrug substrate specificity of liver carboxylesterase
|
|
41032
|
Nucleoside ester prodrug substrate specificity of liver carboxylesterase
|
|
41033
|
Nucleoside ester prodrug substrate specificity of liver carboxylesterase
|
|
41034
|
Nucleoside ester prodrug substrate specificity of liver carboxylesterase
|
|
41035
|
Nucleoside ester prodrug substrate specificity of liver carboxylesterase
|
|
41036
|
Nucleoside ester prodrug substrate specificity of liver carboxylesterase
|
|
41037
|
Nucleoside ester prodrug substrate specificity of liver carboxylesterase
|
|
41038
|
Nucleoside ester prodrug substrate specificity of liver carboxylesterase
|
|
41039
|
Nucleoside ester prodrug substrate specificity of liver carboxylesterase
|
|
41040
|
Nucleoside ester prodrug substrate specificity of liver carboxylesterase
|
|
41041
|
Nucleoside ester prodrug substrate specificity of liver carboxylesterase
|
|
41042
|
Nucleoside ester prodrug substrate specificity of liver carboxylesterase
|
|
41043
|
Nucleoside ester prodrug substrate specificity of liver carboxylesterase
|
|
41044
|
Nucleoside ester prodrug substrate specificity of liver carboxylesterase
|
|
41045
|
Nucleoside ester prodrug substrate specificity of liver carboxylesterase
|
|
41046
|
Nucleoside ester prodrug substrate specificity of liver carboxylesterase
|
|
41047
|
Nucleoside ester prodrug substrate specificity of liver carboxylesterase
|
|
41048
|
Nucleoside ester prodrug substrate specificity of liver carboxylesterase
|
|
41049
|
Nucleoside ester prodrug substrate specificity of liver carboxylesterase
|
|
41050
|
Nucleoside ester prodrug substrate specificity of liver carboxylesterase
|
|
41051
|
Nucleoside ester prodrug substrate specificity of liver carboxylesterase
|
|
41052
|
Nucleoside ester prodrug substrate specificity of liver carboxylesterase
|
|
41053
|
Nucleoside ester prodrug substrate specificity of liver carboxylesterase
|
|
41054
|
Nucleoside ester prodrug substrate specificity of liver carboxylesterase
|
|
41055
|
Nucleoside ester prodrug substrate specificity of liver carboxylesterase
|
|
41056
|
Nucleoside ester prodrug substrate specificity of liver carboxylesterase
|
|
41057
|
Nucleoside ester prodrug substrate specificity of liver carboxylesterase
|
|
41058
|
Nucleoside ester prodrug substrate specificity of liver carboxylesterase
|
|
41059
|
Nucleoside ester prodrug substrate specificity of liver carboxylesterase
|
|
41060
|
Nucleoside ester prodrug substrate specificity of liver carboxylesterase
|
|
41061
|
Efficient purification and molecular properties of spinach chloroplast fructose 1,6-bisphosphatase
|
|
41062
|
Efficient purification and molecular properties of spinach chloroplast fructose 1,6-bisphosphatase
|
|
41063
|
Efficient purification and molecular properties of spinach chloroplast fructose 1,6-bisphosphatase
|
|
41064
|
Efficient purification and molecular properties of spinach chloroplast fructose 1,6-bisphosphatase
|
|
41065
|
Efficient purification and molecular properties of spinach chloroplast fructose 1,6-bisphosphatase
|
|
41066
|
Efficient purification and molecular properties of spinach chloroplast fructose 1,6-bisphosphatase
|
|
41067
|
Efficient purification and molecular properties of spinach chloroplast fructose 1,6-bisphosphatase
|
|
41068
|
Efficient purification and molecular properties of spinach chloroplast fructose 1,6-bisphosphatase
|
|
41069
|
Mechanism of GlvA from Bacillus subtilis: a detailed kinetic analysis of a 6-phospho-alpha-glucosidase from ...
|
|
41070
|
Mechanism of GlvA from Bacillus subtilis: a detailed kinetic analysis of a 6-phospho-alpha-glucosidase from ...
|
|
41071
|
Mechanism of GlvA from Bacillus subtilis: a detailed kinetic analysis of a 6-phospho-alpha-glucosidase from ...
|
|
41072
|
Mechanism of GlvA from Bacillus subtilis: a detailed kinetic analysis of a 6-phospho-alpha-glucosidase from ...
|
|
41073
|
Mechanism of GlvA from Bacillus subtilis: a detailed kinetic analysis of a 6-phospho-alpha-glucosidase from ...
|
|
41074
|
Mechanism of GlvA from Bacillus subtilis: a detailed kinetic analysis of a 6-phospho-alpha-glucosidase from ...
|
|
41075
|
Mechanism of GlvA from Bacillus subtilis: a detailed kinetic analysis of a 6-phospho-alpha-glucosidase from ...
|
|
41076
|
Mechanism of GlvA from Bacillus subtilis: a detailed kinetic analysis of a 6-phospho-alpha-glucosidase from ...
|
|
41077
|
Mechanism of GlvA from Bacillus subtilis: a detailed kinetic analysis of a 6-phospho-alpha-glucosidase from ...
|
|
41078
|
Mechanism of GlvA from Bacillus subtilis: a detailed kinetic analysis of a 6-phospho-alpha-glucosidase from ...
|
|
41079
|
Mechanism of GlvA from Bacillus subtilis: a detailed kinetic analysis of a 6-phospho-alpha-glucosidase from ...
|
|
41080
|
Mechanism of GlvA from Bacillus subtilis: a detailed kinetic analysis of a 6-phospho-alpha-glucosidase from ...
|
|
41081
|
Mechanism of GlvA from Bacillus subtilis: a detailed kinetic analysis of a 6-phospho-alpha-glucosidase from ...
|
|
41082
|
Mechanism of GlvA from Bacillus subtilis: a detailed kinetic analysis of a 6-phospho-alpha-glucosidase from ...
|
|
41083
|
Mechanism of GlvA from Bacillus subtilis: a detailed kinetic analysis of a 6-phospho-alpha-glucosidase from ...
|
|
41084
|
Mechanism of GlvA from Bacillus subtilis: a detailed kinetic analysis of a 6-phospho-alpha-glucosidase from ...
|
|
41085
|
Mechanism of GlvA from Bacillus subtilis: a detailed kinetic analysis of a 6-phospho-alpha-glucosidase from ...
|
|
41086
|
Mechanism of GlvA from Bacillus subtilis: a detailed kinetic analysis of a 6-phospho-alpha-glucosidase from ...
|
|
41087
|
Mechanism of GlvA from Bacillus subtilis: a detailed kinetic analysis of a 6-phospho-alpha-glucosidase from ...
|
|
41088
|
Mechanism of GlvA from Bacillus subtilis: a detailed kinetic analysis of a 6-phospho-alpha-glucosidase from ...
|
|
41089
|
Mechanism of GlvA from Bacillus subtilis: a detailed kinetic analysis of a 6-phospho-alpha-glucosidase from ...
|
|
41090
|
Mechanism of GlvA from Bacillus subtilis: a detailed kinetic analysis of a 6-phospho-alpha-glucosidase from ...
|
|
41091
|
Mechanism of GlvA from Bacillus subtilis: a detailed kinetic analysis of a 6-phospho-alpha-glucosidase from ...
|
|
41092
|
Mechanism of GlvA from Bacillus subtilis: a detailed kinetic analysis of a 6-phospho-alpha-glucosidase from ...
|
|
41093
|
Mechanism of GlvA from Bacillus subtilis: a detailed kinetic analysis of a 6-phospho-alpha-glucosidase from ...
|
|
41094
|
Mechanism of GlvA from Bacillus subtilis: a detailed kinetic analysis of a 6-phospho-alpha-glucosidase from ...
|
|
41095
|
Mechanism of GlvA from Bacillus subtilis: a detailed kinetic analysis of a 6-phospho-alpha-glucosidase from ...
|
|
41096
|
Mechanism of GlvA from Bacillus subtilis: a detailed kinetic analysis of a 6-phospho-alpha-glucosidase from ...
|
|
41097
|
Mechanism of GlvA from Bacillus subtilis: a detailed kinetic analysis of a 6-phospho-alpha-glucosidase from ...
|
|
41098
|
Mechanism of GlvA from Bacillus subtilis: a detailed kinetic analysis of a 6-phospho-alpha-glucosidase from ...
|
|
41099
|
Mechanism of GlvA from Bacillus subtilis: a detailed kinetic analysis of a 6-phospho-alpha-glucosidase from ...
|
|
41100
|
Mechanism of GlvA from Bacillus subtilis: a detailed kinetic analysis of a 6-phospho-alpha-glucosidase from ...
|
|
41101
|
Mechanism of GlvA from Bacillus subtilis: a detailed kinetic analysis of a 6-phospho-alpha-glucosidase from ...
|
|
41102
|
Mechanism of GlvA from Bacillus subtilis: a detailed kinetic analysis of a 6-phospho-alpha-glucosidase from ...
|
|
41103
|
Mechanism of GlvA from Bacillus subtilis: a detailed kinetic analysis of a 6-phospho-alpha-glucosidase from ...
|
|
41104
|
Mechanism of GlvA from Bacillus subtilis: a detailed kinetic analysis of a 6-phospho-alpha-glucosidase from ...
|
|
41105
|
Identification of the catalytic nucleophile in Family 42 beta-galactosidases by intermediate trapping and ...
|
|
41106
|
Identification of the catalytic nucleophile in Family 42 beta-galactosidases by intermediate trapping and ...
|
|
41107
|
Identification of the catalytic nucleophile in Family 42 beta-galactosidases by intermediate trapping and ...
|
|
41108
|
Identification of the catalytic nucleophile in Family 42 beta-galactosidases by intermediate trapping and ...
|
|
41109
|
Structure of the human 3alpha-hydroxysteroid dehydrogenase type 3 in complex with testosterone and NADP at ...
|
|
41110
|
Structure of the human 3alpha-hydroxysteroid dehydrogenase type 3 in complex with testosterone and NADP at ...
|
|
41111
|
Structure of the human 3alpha-hydroxysteroid dehydrogenase type 3 in complex with testosterone and NADP at ...
|
|
41112
|
Yeast phenylalanine ammonia-lyase. Purification, properties, and the identification of catalytically essential ...
|
|
41113
|
Yeast phenylalanine ammonia-lyase. Purification, properties, and the identification of catalytically essential ...
|
|
41114
|
Yeast phenylalanine ammonia-lyase. Purification, properties, and the identification of catalytically essential ...
|
|
41115
|
Yeast phenylalanine ammonia-lyase. Purification, properties, and the identification of catalytically essential ...
|
|
41116
|
Yeast phenylalanine ammonia-lyase. Purification, properties, and the identification of catalytically essential ...
|
|
41117
|
Yeast phenylalanine ammonia-lyase. Purification, properties, and the identification of catalytically essential ...
|
|
41118
|
Yeast phenylalanine ammonia-lyase. Purification, properties, and the identification of catalytically essential ...
|
|
41119
|
Yeast phenylalanine ammonia-lyase. Purification, properties, and the identification of catalytically essential ...
|
|
41120
|
Yeast phenylalanine ammonia-lyase. Purification, properties, and the identification of catalytically essential ...
|
|
41121
|
Yeast phenylalanine ammonia-lyase. Purification, properties, and the identification of catalytically essential ...
|
|
41122
|
Yeast phenylalanine ammonia-lyase. Purification, properties, and the identification of catalytically essential ...
|
|
41123
|
Yeast phenylalanine ammonia-lyase. Purification, properties, and the identification of catalytically essential ...
|
|
41124
|
Yeast phenylalanine ammonia-lyase. Purification, properties, and the identification of catalytically essential ...
|
|
41125
|
Yeast phenylalanine ammonia-lyase. Purification, properties, and the identification of catalytically essential ...
|
|
41126
|
Yeast phenylalanine ammonia-lyase. Purification, properties, and the identification of catalytically essential ...
|
|
41127
|
Adenosine triphosphate-adenosine-5`-monophosphate phosphotransferase from normal human liver mitochondria. ...
|
|
41128
|
Adenosine triphosphate-adenosine-5`-monophosphate phosphotransferase from normal human liver mitochondria. ...
|
|
41129
|
Adenosine triphosphate-adenosine-5`-monophosphate phosphotransferase from normal human liver mitochondria. ...
|
|
41130
|
Adenosine triphosphate-adenosine-5`-monophosphate phosphotransferase from normal human liver mitochondria. ...
|
|
41131
|
An acyl-coenzyme A chain length dependent assay for 3-oxoacyl-coenzyme A thiolases employing ...
|
|
41132
|
An acyl-coenzyme A chain length dependent assay for 3-oxoacyl-coenzyme A thiolases employing ...
|
|
41133
|
An acyl-coenzyme A chain length dependent assay for 3-oxoacyl-coenzyme A thiolases employing ...
|
|
41134
|
An acyl-coenzyme A chain length dependent assay for 3-oxoacyl-coenzyme A thiolases employing ...
|
|
41135
|
An acyl-coenzyme A chain length dependent assay for 3-oxoacyl-coenzyme A thiolases employing ...
|
|
41136
|
An acyl-coenzyme A chain length dependent assay for 3-oxoacyl-coenzyme A thiolases employing ...
|
|
41137
|
An acyl-coenzyme A chain length dependent assay for 3-oxoacyl-coenzyme A thiolases employing ...
|
|
41138
|
An acyl-coenzyme A chain length dependent assay for 3-oxoacyl-coenzyme A thiolases employing ...
|
|
41139
|
An acyl-coenzyme A chain length dependent assay for 3-oxoacyl-coenzyme A thiolases employing ...
|
|
41140
|
An acyl-coenzyme A chain length dependent assay for 3-oxoacyl-coenzyme A thiolases employing ...
|
|
41141
|
An acyl-coenzyme A chain length dependent assay for 3-oxoacyl-coenzyme A thiolases employing ...
|
|
41142
|
An acyl-coenzyme A chain length dependent assay for 3-oxoacyl-coenzyme A thiolases employing ...
|
|
41143
|
An acyl-coenzyme A chain length dependent assay for 3-oxoacyl-coenzyme A thiolases employing ...
|
|
41144
|
An acyl-coenzyme A chain length dependent assay for 3-oxoacyl-coenzyme A thiolases employing ...
|
|
41145
|
An acyl-coenzyme A chain length dependent assay for 3-oxoacyl-coenzyme A thiolases employing ...
|
|
41146
|
An acyl-coenzyme A chain length dependent assay for 3-oxoacyl-coenzyme A thiolases employing ...
|
|
41147
|
An acyl-coenzyme A chain length dependent assay for 3-oxoacyl-coenzyme A thiolases employing ...
|
|
41148
|
An acyl-coenzyme A chain length dependent assay for 3-oxoacyl-coenzyme A thiolases employing ...
|
|
41149
|
An acyl-coenzyme A chain length dependent assay for 3-oxoacyl-coenzyme A thiolases employing ...
|
|
41150
|
Three distinct domains in the cholinesterase molecule confer selectivity for acetyl- and butyrylcholinesterase ...
|
|
41151
|
Three distinct domains in the cholinesterase molecule confer selectivity for acetyl- and butyrylcholinesterase ...
|
|
41152
|
Three distinct domains in the cholinesterase molecule confer selectivity for acetyl- and butyrylcholinesterase ...
|
|
41153
|
Three distinct domains in the cholinesterase molecule confer selectivity for acetyl- and butyrylcholinesterase ...
|
|
41154
|
Three distinct domains in the cholinesterase molecule confer selectivity for acetyl- and butyrylcholinesterase ...
|
|
41155
|
Three distinct domains in the cholinesterase molecule confer selectivity for acetyl- and butyrylcholinesterase ...
|
|
41156
|
Three distinct domains in the cholinesterase molecule confer selectivity for acetyl- and butyrylcholinesterase ...
|
|
41157
|
Three distinct domains in the cholinesterase molecule confer selectivity for acetyl- and butyrylcholinesterase ...
|
|
41158
|
Three distinct domains in the cholinesterase molecule confer selectivity for acetyl- and butyrylcholinesterase ...
|
|
41159
|
Three distinct domains in the cholinesterase molecule confer selectivity for acetyl- and butyrylcholinesterase ...
|
|
41160
|
Three distinct domains in the cholinesterase molecule confer selectivity for acetyl- and butyrylcholinesterase ...
|
|
41161
|
Three distinct domains in the cholinesterase molecule confer selectivity for acetyl- and butyrylcholinesterase ...
|
|
41162
|
Three distinct domains in the cholinesterase molecule confer selectivity for acetyl- and butyrylcholinesterase ...
|
|
41163
|
Three distinct domains in the cholinesterase molecule confer selectivity for acetyl- and butyrylcholinesterase ...
|
|
41164
|
Three distinct domains in the cholinesterase molecule confer selectivity for acetyl- and butyrylcholinesterase ...
|
|
41165
|
Three distinct domains in the cholinesterase molecule confer selectivity for acetyl- and butyrylcholinesterase ...
|
|
41166
|
Three distinct domains in the cholinesterase molecule confer selectivity for acetyl- and butyrylcholinesterase ...
|
|
41167
|
Three distinct domains in the cholinesterase molecule confer selectivity for acetyl- and butyrylcholinesterase ...
|
|
41168
|
Three distinct domains in the cholinesterase molecule confer selectivity for acetyl- and butyrylcholinesterase ...
|
|
41169
|
Three distinct domains in the cholinesterase molecule confer selectivity for acetyl- and butyrylcholinesterase ...
|
|
41170
|
Three distinct domains in the cholinesterase molecule confer selectivity for acetyl- and butyrylcholinesterase ...
|
|
41171
|
Three distinct domains in the cholinesterase molecule confer selectivity for acetyl- and butyrylcholinesterase ...
|
|
41172
|
Three distinct domains in the cholinesterase molecule confer selectivity for acetyl- and butyrylcholinesterase ...
|
|
41173
|
Three distinct domains in the cholinesterase molecule confer selectivity for acetyl- and butyrylcholinesterase ...
|
|
41174
|
Three distinct domains in the cholinesterase molecule confer selectivity for acetyl- and butyrylcholinesterase ...
|
|
41175
|
Three distinct domains in the cholinesterase molecule confer selectivity for acetyl- and butyrylcholinesterase ...
|
|
41176
|
Three distinct domains in the cholinesterase molecule confer selectivity for acetyl- and butyrylcholinesterase ...
|
|
41177
|
Three distinct domains in the cholinesterase molecule confer selectivity for acetyl- and butyrylcholinesterase ...
|
|
41178
|
Three distinct domains in the cholinesterase molecule confer selectivity for acetyl- and butyrylcholinesterase ...
|
|
41179
|
Three distinct domains in the cholinesterase molecule confer selectivity for acetyl- and butyrylcholinesterase ...
|
|
41180
|
Three distinct domains in the cholinesterase molecule confer selectivity for acetyl- and butyrylcholinesterase ...
|
|
41181
|
Three distinct domains in the cholinesterase molecule confer selectivity for acetyl- and butyrylcholinesterase ...
|
|
41182
|
Three distinct domains in the cholinesterase molecule confer selectivity for acetyl- and butyrylcholinesterase ...
|
|
41183
|
Three distinct domains in the cholinesterase molecule confer selectivity for acetyl- and butyrylcholinesterase ...
|
|
41184
|
Three distinct domains in the cholinesterase molecule confer selectivity for acetyl- and butyrylcholinesterase ...
|
|
41185
|
Three distinct domains in the cholinesterase molecule confer selectivity for acetyl- and butyrylcholinesterase ...
|
|
41186
|
Three distinct domains in the cholinesterase molecule confer selectivity for acetyl- and butyrylcholinesterase ...
|
|
41187
|
Three distinct domains in the cholinesterase molecule confer selectivity for acetyl- and butyrylcholinesterase ...
|
|
41188
|
Three distinct domains in the cholinesterase molecule confer selectivity for acetyl- and butyrylcholinesterase ...
|
|
41189
|
Three distinct domains in the cholinesterase molecule confer selectivity for acetyl- and butyrylcholinesterase ...
|
|
41190
|
Three distinct domains in the cholinesterase molecule confer selectivity for acetyl- and butyrylcholinesterase ...
|
|
41191
|
Three distinct domains in the cholinesterase molecule confer selectivity for acetyl- and butyrylcholinesterase ...
|
|
41192
|
Three distinct domains in the cholinesterase molecule confer selectivity for acetyl- and butyrylcholinesterase ...
|
|
41193
|
Three distinct domains in the cholinesterase molecule confer selectivity for acetyl- and butyrylcholinesterase ...
|
|
41194
|
Three distinct domains in the cholinesterase molecule confer selectivity for acetyl- and butyrylcholinesterase ...
|
|
41195
|
Three distinct domains in the cholinesterase molecule confer selectivity for acetyl- and butyrylcholinesterase ...
|
|
41196
|
Three distinct domains in the cholinesterase molecule confer selectivity for acetyl- and butyrylcholinesterase ...
|
|
41197
|
Three distinct domains in the cholinesterase molecule confer selectivity for acetyl- and butyrylcholinesterase ...
|
|
41198
|
Three distinct domains in the cholinesterase molecule confer selectivity for acetyl- and butyrylcholinesterase ...
|
|
41199
|
Three distinct domains in the cholinesterase molecule confer selectivity for acetyl- and butyrylcholinesterase ...
|
|
41200
|
Three distinct domains in the cholinesterase molecule confer selectivity for acetyl- and butyrylcholinesterase ...
|
|
41201
|
Three distinct domains in the cholinesterase molecule confer selectivity for acetyl- and butyrylcholinesterase ...
|
|
41202
|
Three distinct domains in the cholinesterase molecule confer selectivity for acetyl- and butyrylcholinesterase ...
|
|
41203
|
Three distinct domains in the cholinesterase molecule confer selectivity for acetyl- and butyrylcholinesterase ...
|
|
41204
|
Three distinct domains in the cholinesterase molecule confer selectivity for acetyl- and butyrylcholinesterase ...
|
|
41205
|
Three distinct domains in the cholinesterase molecule confer selectivity for acetyl- and butyrylcholinesterase ...
|
|
41206
|
Three distinct domains in the cholinesterase molecule confer selectivity for acetyl- and butyrylcholinesterase ...
|
|
41207
|
Three distinct domains in the cholinesterase molecule confer selectivity for acetyl- and butyrylcholinesterase ...
|
|
41208
|
Three distinct domains in the cholinesterase molecule confer selectivity for acetyl- and butyrylcholinesterase ...
|
|
41209
|
Three distinct domains in the cholinesterase molecule confer selectivity for acetyl- and butyrylcholinesterase ...
|
|
41210
|
Three distinct domains in the cholinesterase molecule confer selectivity for acetyl- and butyrylcholinesterase ...
|
|
41211
|
Three distinct domains in the cholinesterase molecule confer selectivity for acetyl- and butyrylcholinesterase ...
|
|
41212
|
Three distinct domains in the cholinesterase molecule confer selectivity for acetyl- and butyrylcholinesterase ...
|
|
41213
|
Three distinct domains in the cholinesterase molecule confer selectivity for acetyl- and butyrylcholinesterase ...
|
|
41214
|
Three distinct domains in the cholinesterase molecule confer selectivity for acetyl- and butyrylcholinesterase ...
|
|
41215
|
Three distinct domains in the cholinesterase molecule confer selectivity for acetyl- and butyrylcholinesterase ...
|
|
41216
|
Three distinct domains in the cholinesterase molecule confer selectivity for acetyl- and butyrylcholinesterase ...
|
|
41217
|
Three distinct domains in the cholinesterase molecule confer selectivity for acetyl- and butyrylcholinesterase ...
|
|
41218
|
Three distinct domains in the cholinesterase molecule confer selectivity for acetyl- and butyrylcholinesterase ...
|
|
41219
|
Three distinct domains in the cholinesterase molecule confer selectivity for acetyl- and butyrylcholinesterase ...
|
|
41220
|
Three distinct domains in the cholinesterase molecule confer selectivity for acetyl- and butyrylcholinesterase ...
|
|
41221
|
Three distinct domains in the cholinesterase molecule confer selectivity for acetyl- and butyrylcholinesterase ...
|
|
41222
|
Three distinct domains in the cholinesterase molecule confer selectivity for acetyl- and butyrylcholinesterase ...
|
|
41223
|
Three distinct domains in the cholinesterase molecule confer selectivity for acetyl- and butyrylcholinesterase ...
|
|
41224
|
Three distinct domains in the cholinesterase molecule confer selectivity for acetyl- and butyrylcholinesterase ...
|
|
41225
|
Three distinct domains in the cholinesterase molecule confer selectivity for acetyl- and butyrylcholinesterase ...
|
|
41226
|
Three distinct domains in the cholinesterase molecule confer selectivity for acetyl- and butyrylcholinesterase ...
|
|
41227
|
Three distinct domains in the cholinesterase molecule confer selectivity for acetyl- and butyrylcholinesterase ...
|
|
41228
|
Three distinct domains in the cholinesterase molecule confer selectivity for acetyl- and butyrylcholinesterase ...
|
|
41229
|
Three distinct domains in the cholinesterase molecule confer selectivity for acetyl- and butyrylcholinesterase ...
|
|
41230
|
Three distinct domains in the cholinesterase molecule confer selectivity for acetyl- and butyrylcholinesterase ...
|
|
41231
|
Three distinct domains in the cholinesterase molecule confer selectivity for acetyl- and butyrylcholinesterase ...
|
|
41232
|
Three distinct domains in the cholinesterase molecule confer selectivity for acetyl- and butyrylcholinesterase ...
|
|
41233
|
Three distinct domains in the cholinesterase molecule confer selectivity for acetyl- and butyrylcholinesterase ...
|
|
41234
|
Three distinct domains in the cholinesterase molecule confer selectivity for acetyl- and butyrylcholinesterase ...
|
|
41235
|
Three distinct domains in the cholinesterase molecule confer selectivity for acetyl- and butyrylcholinesterase ...
|
|
41236
|
Three distinct domains in the cholinesterase molecule confer selectivity for acetyl- and butyrylcholinesterase ...
|
|
41237
|
Three distinct domains in the cholinesterase molecule confer selectivity for acetyl- and butyrylcholinesterase ...
|
|
41238
|
Three distinct domains in the cholinesterase molecule confer selectivity for acetyl- and butyrylcholinesterase ...
|
|
41239
|
Three distinct domains in the cholinesterase molecule confer selectivity for acetyl- and butyrylcholinesterase ...
|
|
41240
|
Three distinct domains in the cholinesterase molecule confer selectivity for acetyl- and butyrylcholinesterase ...
|
|
41241
|
Three distinct domains in the cholinesterase molecule confer selectivity for acetyl- and butyrylcholinesterase ...
|
|
41242
|
Three distinct domains in the cholinesterase molecule confer selectivity for acetyl- and butyrylcholinesterase ...
|
|
41243
|
Three distinct domains in the cholinesterase molecule confer selectivity for acetyl- and butyrylcholinesterase ...
|
|
41244
|
Three distinct domains in the cholinesterase molecule confer selectivity for acetyl- and butyrylcholinesterase ...
|
|
41245
|
Three distinct domains in the cholinesterase molecule confer selectivity for acetyl- and butyrylcholinesterase ...
|
|
41246
|
Three distinct domains in the cholinesterase molecule confer selectivity for acetyl- and butyrylcholinesterase ...
|
|
41247
|
Three distinct domains in the cholinesterase molecule confer selectivity for acetyl- and butyrylcholinesterase ...
|
|
41248
|
Three distinct domains in the cholinesterase molecule confer selectivity for acetyl- and butyrylcholinesterase ...
|
|
41249
|
Three distinct domains in the cholinesterase molecule confer selectivity for acetyl- and butyrylcholinesterase ...
|
|
41250
|
Three distinct domains in the cholinesterase molecule confer selectivity for acetyl- and butyrylcholinesterase ...
|
|
41251
|
Three distinct domains in the cholinesterase molecule confer selectivity for acetyl- and butyrylcholinesterase ...
|
|
41252
|
Three distinct domains in the cholinesterase molecule confer selectivity for acetyl- and butyrylcholinesterase ...
|
|
41253
|
Three distinct domains in the cholinesterase molecule confer selectivity for acetyl- and butyrylcholinesterase ...
|
|
41254
|
Three distinct domains in the cholinesterase molecule confer selectivity for acetyl- and butyrylcholinesterase ...
|
|
41255
|
Three distinct domains in the cholinesterase molecule confer selectivity for acetyl- and butyrylcholinesterase ...
|
|
41256
|
Three distinct domains in the cholinesterase molecule confer selectivity for acetyl- and butyrylcholinesterase ...
|
|
41257
|
Three distinct domains in the cholinesterase molecule confer selectivity for acetyl- and butyrylcholinesterase ...
|
|
41258
|
Three distinct domains in the cholinesterase molecule confer selectivity for acetyl- and butyrylcholinesterase ...
|
|
41259
|
Three distinct domains in the cholinesterase molecule confer selectivity for acetyl- and butyrylcholinesterase ...
|
|
41260
|
The kinetics, substrate and inhibitor specificity of the lactate transporter of Ehrlich-Lettre tumour cells ...
|
|
41261
|
The kinetics, substrate and inhibitor specificity of the lactate transporter of Ehrlich-Lettre tumour cells ...
|
|
41262
|
The kinetics, substrate and inhibitor specificity of the lactate transporter of Ehrlich-Lettre tumour cells ...
|
|
41263
|
The kinetics, substrate and inhibitor specificity of the lactate transporter of Ehrlich-Lettre tumour cells ...
|
|
41264
|
The kinetics, substrate and inhibitor specificity of the lactate transporter of Ehrlich-Lettre tumour cells ...
|
|
41265
|
The kinetics, substrate and inhibitor specificity of the lactate transporter of Ehrlich-Lettre tumour cells ...
|
|
41266
|
The kinetics, substrate and inhibitor specificity of the lactate transporter of Ehrlich-Lettre tumour cells ...
|
|
41267
|
The kinetics, substrate and inhibitor specificity of the lactate transporter of Ehrlich-Lettre tumour cells ...
|
|
41268
|
The kinetics, substrate and inhibitor specificity of the lactate transporter of Ehrlich-Lettre tumour cells ...
|
|
41269
|
The kinetics, substrate and inhibitor specificity of the lactate transporter of Ehrlich-Lettre tumour cells ...
|
|
41270
|
The kinetics, substrate and inhibitor specificity of the lactate transporter of Ehrlich-Lettre tumour cells ...
|
|
41271
|
The kinetics, substrate and inhibitor specificity of the lactate transporter of Ehrlich-Lettre tumour cells ...
|
|
41272
|
The kinetics, substrate and inhibitor specificity of the lactate transporter of Ehrlich-Lettre tumour cells ...
|
|
41273
|
The kinetics, substrate and inhibitor specificity of the lactate transporter of Ehrlich-Lettre tumour cells ...
|
|
41274
|
The kinetics, substrate and inhibitor specificity of the lactate transporter of Ehrlich-Lettre tumour cells ...
|
|
41275
|
The kinetics, substrate and inhibitor specificity of the lactate transporter of Ehrlich-Lettre tumour cells ...
|
|
41276
|
The kinetics, substrate and inhibitor specificity of the lactate transporter of Ehrlich-Lettre tumour cells ...
|
|
41277
|
The kinetics, substrate and inhibitor specificity of the lactate transporter of Ehrlich-Lettre tumour cells ...
|
|
41278
|
The kinetics, substrate and inhibitor specificity of the lactate transporter of Ehrlich-Lettre tumour cells ...
|
|
41279
|
The kinetics, substrate and inhibitor specificity of the lactate transporter of Ehrlich-Lettre tumour cells ...
|
|
41280
|
The kinetics, substrate and inhibitor specificity of the lactate transporter of Ehrlich-Lettre tumour cells ...
|
|
41281
|
The kinetics, substrate and inhibitor specificity of the lactate transporter of Ehrlich-Lettre tumour cells ...
|
|
41282
|
The kinetics, substrate and inhibitor specificity of the lactate transporter of Ehrlich-Lettre tumour cells ...
|
|
41283
|
The kinetics, substrate and inhibitor specificity of the lactate transporter of Ehrlich-Lettre tumour cells ...
|
|
41284
|
The kinetics, substrate and inhibitor specificity of the lactate transporter of Ehrlich-Lettre tumour cells ...
|
|
41285
|
The kinetics, substrate and inhibitor specificity of the lactate transporter of Ehrlich-Lettre tumour cells ...
|
|
41286
|
The kinetics, substrate and inhibitor specificity of the lactate transporter of Ehrlich-Lettre tumour cells ...
|
|
41287
|
The kinetics, substrate and inhibitor specificity of the lactate transporter of Ehrlich-Lettre tumour cells ...
|
|
41288
|
The kinetics, substrate and inhibitor specificity of the lactate transporter of Ehrlich-Lettre tumour cells ...
|
|
41289
|
The kinetics, substrate and inhibitor specificity of the lactate transporter of Ehrlich-Lettre tumour cells ...
|
|
41290
|
The kinetics, substrate and inhibitor specificity of the lactate transporter of Ehrlich-Lettre tumour cells ...
|
|
41291
|
The kinetics, substrate and inhibitor specificity of the lactate transporter of Ehrlich-Lettre tumour cells ...
|
|
41292
|
The kinetics, substrate and inhibitor specificity of the lactate transporter of Ehrlich-Lettre tumour cells ...
|
|
41293
|
The kinetics, substrate and inhibitor specificity of the lactate transporter of Ehrlich-Lettre tumour cells ...
|
|
41294
|
The kinetics, substrate and inhibitor specificity of the lactate transporter of Ehrlich-Lettre tumour cells ...
|
|
41295
|
The kinetics, substrate and inhibitor specificity of the lactate transporter of Ehrlich-Lettre tumour cells ...
|
|
41296
|
The kinetics, substrate and inhibitor specificity of the lactate transporter of Ehrlich-Lettre tumour cells ...
|
|
41297
|
The kinetics, substrate and inhibitor specificity of the lactate transporter of Ehrlich-Lettre tumour cells ...
|
|
41298
|
The kinetics, substrate and inhibitor specificity of the lactate transporter of Ehrlich-Lettre tumour cells ...
|
|
41299
|
The kinetics, substrate and inhibitor specificity of the lactate transporter of Ehrlich-Lettre tumour cells ...
|
|
41300
|
The kinetics, substrate and inhibitor specificity of the lactate transporter of Ehrlich-Lettre tumour cells ...
|
|
41301
|
The kinetics, substrate and inhibitor specificity of the lactate transporter of Ehrlich-Lettre tumour cells ...
|
|
41302
|
The kinetics, substrate and inhibitor specificity of the lactate transporter of Ehrlich-Lettre tumour cells ...
|
|
41303
|
The kinetics, substrate and inhibitor specificity of the lactate transporter of Ehrlich-Lettre tumour cells ...
|
|
41304
|
The kinetics, substrate and inhibitor specificity of the lactate transporter of Ehrlich-Lettre tumour cells ...
|
|
41305
|
The kinetics, substrate and inhibitor specificity of the lactate transporter of Ehrlich-Lettre tumour cells ...
|
|
41306
|
The kinetics, substrate and inhibitor specificity of the lactate transporter of Ehrlich-Lettre tumour cells ...
|
|
41307
|
The kinetics, substrate and inhibitor specificity of the lactate transporter of Ehrlich-Lettre tumour cells ...
|
|
41308
|
The kinetics, substrate, and inhibitor specificity of the monocarboxylate (lactate) transporter of rat liver ...
|
|
41309
|
The kinetics, substrate, and inhibitor specificity of the monocarboxylate (lactate) transporter of rat liver ...
|
|
41310
|
The kinetics, substrate, and inhibitor specificity of the monocarboxylate (lactate) transporter of rat liver ...
|
|
41311
|
The kinetics, substrate, and inhibitor specificity of the monocarboxylate (lactate) transporter of rat liver ...
|
|
41312
|
The kinetics, substrate, and inhibitor specificity of the monocarboxylate (lactate) transporter of rat liver ...
|
|
41313
|
The kinetics, substrate, and inhibitor specificity of the monocarboxylate (lactate) transporter of rat liver ...
|
|
41314
|
The kinetics, substrate, and inhibitor specificity of the monocarboxylate (lactate) transporter of rat liver ...
|
|
41315
|
The kinetics, substrate, and inhibitor specificity of the monocarboxylate (lactate) transporter of rat liver ...
|
|
41316
|
The kinetics, substrate, and inhibitor specificity of the monocarboxylate (lactate) transporter of rat liver ...
|
|
41317
|
The kinetics, substrate, and inhibitor specificity of the monocarboxylate (lactate) transporter of rat liver ...
|
|
41318
|
The kinetics, substrate, and inhibitor specificity of the monocarboxylate (lactate) transporter of rat liver ...
|
|
41319
|
The kinetics, substrate, and inhibitor specificity of the monocarboxylate (lactate) transporter of rat liver ...
|
|
41320
|
The kinetics, substrate, and inhibitor specificity of the monocarboxylate (lactate) transporter of rat liver ...
|
|
41321
|
The kinetics, substrate, and inhibitor specificity of the monocarboxylate (lactate) transporter of rat liver ...
|
|
41322
|
The kinetics, substrate, and inhibitor specificity of the monocarboxylate (lactate) transporter of rat liver ...
|
|
41323
|
The kinetics, substrate, and inhibitor specificity of the monocarboxylate (lactate) transporter of rat liver ...
|
|
41324
|
The kinetics, substrate, and inhibitor specificity of the monocarboxylate (lactate) transporter of rat liver ...
|
|
41325
|
The kinetics, substrate, and inhibitor specificity of the monocarboxylate (lactate) transporter of rat liver ...
|
|
41326
|
The kinetics, substrate, and inhibitor specificity of the monocarboxylate (lactate) transporter of rat liver ...
|
|
41327
|
The kinetics, substrate, and inhibitor specificity of the monocarboxylate (lactate) transporter of rat liver ...
|
|
41328
|
The kinetics, substrate, and inhibitor specificity of the monocarboxylate (lactate) transporter of rat liver ...
|
|
41329
|
The kinetics, substrate, and inhibitor specificity of the monocarboxylate (lactate) transporter of rat liver ...
|
|
41330
|
The kinetics, substrate, and inhibitor specificity of the monocarboxylate (lactate) transporter of rat liver ...
|
|
41331
|
The kinetics, substrate, and inhibitor specificity of the monocarboxylate (lactate) transporter of rat liver ...
|
|
41332
|
The kinetics, substrate, and inhibitor specificity of the monocarboxylate (lactate) transporter of rat liver ...
|
|
41333
|
The kinetics, substrate, and inhibitor specificity of the monocarboxylate (lactate) transporter of rat liver ...
|
|
41334
|
The kinetics, substrate, and inhibitor specificity of the monocarboxylate (lactate) transporter of rat liver ...
|
|
41335
|
The kinetics, substrate, and inhibitor specificity of the monocarboxylate (lactate) transporter of rat liver ...
|
|
41336
|
The kinetics, substrate, and inhibitor specificity of the monocarboxylate (lactate) transporter of rat liver ...
|
|
41337
|
The kinetics, substrate, and inhibitor specificity of the monocarboxylate (lactate) transporter of rat liver ...
|
|
41338
|
The kinetics, substrate, and inhibitor specificity of the monocarboxylate (lactate) transporter of rat liver ...
|
|
41339
|
The kinetics, substrate, and inhibitor specificity of the monocarboxylate (lactate) transporter of rat liver ...
|
|
41340
|
The kinetics, substrate, and inhibitor specificity of the monocarboxylate (lactate) transporter of rat liver ...
|
|
41341
|
The kinetics, substrate, and inhibitor specificity of the monocarboxylate (lactate) transporter of rat liver ...
|
|
41342
|
The kinetics, substrate, and inhibitor specificity of the monocarboxylate (lactate) transporter of rat liver ...
|
|
41343
|
The kinetics, substrate, and inhibitor specificity of the monocarboxylate (lactate) transporter of rat liver ...
|
|
41344
|
The kinetics, substrate, and inhibitor specificity of the monocarboxylate (lactate) transporter of rat liver ...
|
|
41345
|
Characterization of the inactivation of rat fatty acid synthase by C75: Inhibition of partial reactions and ...
|
|
41346
|
Characterization of the inactivation of rat fatty acid synthase by C75: Inhibition of partial reactions and ...
|
|
41347
|
Characterization of the inactivation of rat fatty acid synthase by C75: Inhibition of partial reactions and ...
|
|
41348
|
Characterization of the inactivation of rat fatty acid synthase by C75: Inhibition of partial reactions and ...
|
|
41349
|
Identification of a catalytic residue of Clostridium paraputrificum N-acetyl-beta-D-glucosaminidase Nag3A by ...
|
|
41350
|
Identification of a catalytic residue of Clostridium paraputrificum N-acetyl-beta-D-glucosaminidase Nag3A by ...
|
|
41351
|
Identification of a catalytic residue of Clostridium paraputrificum N-acetyl-beta-D-glucosaminidase Nag3A by ...
|
|
41352
|
Identification of a catalytic residue of Clostridium paraputrificum N-acetyl-beta-D-glucosaminidase Nag3A by ...
|
|
41353
|
Identification of a catalytic residue of Clostridium paraputrificum N-acetyl-beta-D-glucosaminidase Nag3A by ...
|
|
41354
|
Identification of a catalytic residue of Clostridium paraputrificum N-acetyl-beta-D-glucosaminidase Nag3A by ...
|
|
41355
|
Identification of a catalytic residue of Clostridium paraputrificum N-acetyl-beta-D-glucosaminidase Nag3A by ...
|
|
41356
|
Identification of a catalytic residue of Clostridium paraputrificum N-acetyl-beta-D-glucosaminidase Nag3A by ...
|
|
41357
|
Identification of a catalytic residue of Clostridium paraputrificum N-acetyl-beta-D-glucosaminidase Nag3A by ...
|
|
41358
|
Identification of a catalytic residue of Clostridium paraputrificum N-acetyl-beta-D-glucosaminidase Nag3A by ...
|
|
41359
|
Rat inositol 1,4,5-trisphosphate 3-kinase C is enzymatically specialized for basal cellular inositol ...
|
|
41360
|
Rat inositol 1,4,5-trisphosphate 3-kinase C is enzymatically specialized for basal cellular inositol ...
|
|
41361
|
Rat inositol 1,4,5-trisphosphate 3-kinase C is enzymatically specialized for basal cellular inositol ...
|
|
41362
|
Rat inositol 1,4,5-trisphosphate 3-kinase C is enzymatically specialized for basal cellular inositol ...
|
|
41363
|
Rat inositol 1,4,5-trisphosphate 3-kinase C is enzymatically specialized for basal cellular inositol ...
|
|
41364
|
Rat inositol 1,4,5-trisphosphate 3-kinase C is enzymatically specialized for basal cellular inositol ...
|
|
41365
|
Rat inositol 1,4,5-trisphosphate 3-kinase C is enzymatically specialized for basal cellular inositol ...
|
|
41366
|
Rat inositol 1,4,5-trisphosphate 3-kinase C is enzymatically specialized for basal cellular inositol ...
|
|
41367
|
Rat inositol 1,4,5-trisphosphate 3-kinase C is enzymatically specialized for basal cellular inositol ...
|
|
41368
|
Rat inositol 1,4,5-trisphosphate 3-kinase C is enzymatically specialized for basal cellular inositol ...
|
|
41369
|
Rat inositol 1,4,5-trisphosphate 3-kinase C is enzymatically specialized for basal cellular inositol ...
|
|
41370
|
Rat inositol 1,4,5-trisphosphate 3-kinase C is enzymatically specialized for basal cellular inositol ...
|
|
41371
|
Rat inositol 1,4,5-trisphosphate 3-kinase C is enzymatically specialized for basal cellular inositol ...
|
|
41372
|
Rat inositol 1,4,5-trisphosphate 3-kinase C is enzymatically specialized for basal cellular inositol ...
|
|
41373
|
Rat inositol 1,4,5-trisphosphate 3-kinase C is enzymatically specialized for basal cellular inositol ...
|
|
41374
|
Rat inositol 1,4,5-trisphosphate 3-kinase C is enzymatically specialized for basal cellular inositol ...
|
|
41375
|
Rat inositol 1,4,5-trisphosphate 3-kinase C is enzymatically specialized for basal cellular inositol ...
|
|
41376
|
Rat inositol 1,4,5-trisphosphate 3-kinase C is enzymatically specialized for basal cellular inositol ...
|
|
41377
|
Biosynthesis of truncated N-linked oligosaccharides results from non-orthologous hexosaminidase-mediated ...
|
|
41378
|
Biosynthesis of truncated N-linked oligosaccharides results from non-orthologous hexosaminidase-mediated ...
|
|
41379
|
Biosynthesis of truncated N-linked oligosaccharides results from non-orthologous hexosaminidase-mediated ...
|
|
41380
|
Biosynthesis of truncated N-linked oligosaccharides results from non-orthologous hexosaminidase-mediated ...
|
|
41381
|
Peroxisomes contain a specific phytanoyl-CoA/pristanoyl-CoA thioesterase acting as a novel auxiliary enzyme in ...
|
|
41382
|
Peroxisomes contain a specific phytanoyl-CoA/pristanoyl-CoA thioesterase acting as a novel auxiliary enzyme in ...
|
|
41383
|
Peroxisomes contain a specific phytanoyl-CoA/pristanoyl-CoA thioesterase acting as a novel auxiliary enzyme in ...
|
|
41384
|
Hydroxyatrazine N-ethylaminohydrolase (AtzB): an amidohydrolase superfamily enzyme catalyzing deamination and ...
|
|
41385
|
Hydroxyatrazine N-ethylaminohydrolase (AtzB): an amidohydrolase superfamily enzyme catalyzing deamination and ...
|
|
41386
|
Hydroxyatrazine N-ethylaminohydrolase (AtzB): an amidohydrolase superfamily enzyme catalyzing deamination and ...
|
|
41387
|
Hydroxyatrazine N-ethylaminohydrolase (AtzB): an amidohydrolase superfamily enzyme catalyzing deamination and ...
|
|
41388
|
Hydroxyatrazine N-ethylaminohydrolase (AtzB): an amidohydrolase superfamily enzyme catalyzing deamination and ...
|
|
41389
|
The purification and properties of myo-inositol monophosphatase from bovine brain.
|
|
41390
|
The purification and properties of myo-inositol monophosphatase from bovine brain.
|
|
41391
|
The purification and properties of myo-inositol monophosphatase from bovine brain.
|
|
41392
|
The purification and properties of myo-inositol monophosphatase from bovine brain.
|
|
41393
|
The purification and properties of myo-inositol monophosphatase from bovine brain.
|
|
41394
|
The purification and properties of myo-inositol monophosphatase from bovine brain.
|
|
41395
|
The purification and properties of myo-inositol monophosphatase from bovine brain.
|
|
41396
|
The purification and properties of myo-inositol monophosphatase from bovine brain.
|
|
41397
|
The purification and properties of myo-inositol monophosphatase from bovine brain.
|
|
41398
|
The purification and properties of myo-inositol monophosphatase from bovine brain.
|
|
41399
|
Aerobic benzoyl-coenzyme A (CoA) catabolic pathway in Azoarcus evansii: conversion of ring cleavage product by ...
|
|
41400
|
Identification of lactaldehyde dehydrogenase in Methanocaldococcus jannaschii and its involvement in ...
|
|
41401
|
Identification of lactaldehyde dehydrogenase in Methanocaldococcus jannaschii and its involvement in ...
|
|
41402
|
The metabolism of L-fucose. II. The enzymatic cleavage of L-fuculose 1-phosphate
|
|
41403
|
Malolactic enzyme of Lactobacillus plantarum. Purification, properties, and distribution among bacteria
|
|
41404
|
Malolactic enzyme of Lactobacillus plantarum. Purification, properties, and distribution among bacteria
|
|
41405
|
Malolactic enzyme of Lactobacillus plantarum. Purification, properties, and distribution among bacteria
|
|
41406
|
Intramolecular regulation of the opposing (p)ppGpp catalytic activities of Rel(Seq), the Rel/Spo enzyme from ...
|
|
41407
|
Functional characterization of genetic variants of human FMO3 associated with trimethylaminuria
|
|
41408
|
Functional characterization of genetic variants of human FMO3 associated with trimethylaminuria
|
|
41409
|
Functional characterization of genetic variants of human FMO3 associated with trimethylaminuria
|
|
41410
|
Functional characterization of genetic variants of human FMO3 associated with trimethylaminuria
|
|
41411
|
Functional characterization of genetic variants of human FMO3 associated with trimethylaminuria
|
|
41412
|
Functional characterization of genetic variants of human FMO3 associated with trimethylaminuria
|
|
41413
|
Functional characterization of genetic variants of human FMO3 associated with trimethylaminuria
|
|
41414
|
Functional characterization of genetic variants of human FMO3 associated with trimethylaminuria
|
|
41415
|
Functional characterization of genetic variants of human FMO3 associated with trimethylaminuria
|
|
41416
|
Functional characterization of genetic variants of human FMO3 associated with trimethylaminuria
|
|
41417
|
Functional characterization of genetic variants of human FMO3 associated with trimethylaminuria
|
|
41418
|
Functional characterization of genetic variants of human FMO3 associated with trimethylaminuria
|
|
41419
|
Functional characterization of genetic variants of human FMO3 associated with trimethylaminuria
|
|
41420
|
Functional characterization of genetic variants of human FMO3 associated with trimethylaminuria
|
|
41421
|
Functional characterization of genetic variants of human FMO3 associated with trimethylaminuria
|
|
41422
|
Functional characterization of genetic variants of human FMO3 associated with trimethylaminuria
|
|
41423
|
Functional characterization of genetic variants of human FMO3 associated with trimethylaminuria
|
|
41424
|
Functional characterization of genetic variants of human FMO3 associated with trimethylaminuria
|
|
41425
|
Functional characterization of genetic variants of human FMO3 associated with trimethylaminuria
|
|
41426
|
Functional characterization of genetic variants of human FMO3 associated with trimethylaminuria
|
|
41427
|
Functional characterization of genetic variants of human FMO3 associated with trimethylaminuria
|
|
41428
|
Influence of water-miscible organic solvents on kinetics and enantioselectivity of the (R)-specific alcohol ...
|
|
41429
|
Influence of water-miscible organic solvents on kinetics and enantioselectivity of the (R)-specific alcohol ...
|
|
41430
|
Influence of water-miscible organic solvents on kinetics and enantioselectivity of the (R)-specific alcohol ...
|
|
41431
|
Influence of water-miscible organic solvents on kinetics and enantioselectivity of the (R)-specific alcohol ...
|
|
41432
|
Influence of water-miscible organic solvents on kinetics and enantioselectivity of the (R)-specific alcohol ...
|
|
41433
|
Influence of water-miscible organic solvents on kinetics and enantioselectivity of the (R)-specific alcohol ...
|
|
41434
|
Influence of water-miscible organic solvents on kinetics and enantioselectivity of the (R)-specific alcohol ...
|
|
41435
|
Influence of water-miscible organic solvents on kinetics and enantioselectivity of the (R)-specific alcohol ...
|
|
41436
|
Influence of water-miscible organic solvents on kinetics and enantioselectivity of the (R)-specific alcohol ...
|
|
41437
|
The macrophage migration inhibitory factor MIF is a phenylpyruvate tautomerase
|
|
41438
|
The macrophage migration inhibitory factor MIF is a phenylpyruvate tautomerase
|
|
41439
|
Evidence that human histidine triad nucleotide binding protein 3 (Hint3) is a distinct branch of the histidine ...
|
|
41440
|
Evidence that human histidine triad nucleotide binding protein 3 (Hint3) is a distinct branch of the histidine ...
|
|
41441
|
Evidence that human histidine triad nucleotide binding protein 3 (Hint3) is a distinct branch of the histidine ...
|
|
41442
|
Evidence that human histidine triad nucleotide binding protein 3 (Hint3) is a distinct branch of the histidine ...
|
|
41443
|
Evidence that human histidine triad nucleotide binding protein 3 (Hint3) is a distinct branch of the histidine ...
|
|
41444
|
Evidence that human histidine triad nucleotide binding protein 3 (Hint3) is a distinct branch of the histidine ...
|
|
41445
|
Evidence that human histidine triad nucleotide binding protein 3 (Hint3) is a distinct branch of the histidine ...
|
|
41446
|
Evidence that human histidine triad nucleotide binding protein 3 (Hint3) is a distinct branch of the histidine ...
|
|
41447
|
Evidence that human histidine triad nucleotide binding protein 3 (Hint3) is a distinct branch of the histidine ...
|
|
41448
|
Evidence that human histidine triad nucleotide binding protein 3 (Hint3) is a distinct branch of the histidine ...
|
|
41449
|
Evidence that human histidine triad nucleotide binding protein 3 (Hint3) is a distinct branch of the histidine ...
|
|
41450
|
Evidence that human histidine triad nucleotide binding protein 3 (Hint3) is a distinct branch of the histidine ...
|
|
41451
|
C-terminal end and aminoacid Lys48 in HMG-CoA lyase are involved in substrate binding and enzyme activity
|
|
41452
|
C-terminal end and aminoacid Lys48 in HMG-CoA lyase are involved in substrate binding and enzyme activity
|
|
41453
|
Determination of specific activities and kinetic constants of biotinidase and lipoamidase in LEW rat and ...
|
|
41454
|
Determination of specific activities and kinetic constants of biotinidase and lipoamidase in LEW rat and ...
|
|
41455
|
Determination of specific activities and kinetic constants of biotinidase and lipoamidase in LEW rat and ...
|
|
41456
|
Determination of specific activities and kinetic constants of biotinidase and lipoamidase in LEW rat and ...
|
|
41457
|
Determination of specific activities and kinetic constants of biotinidase and lipoamidase in LEW rat and ...
|
|
41458
|
Determination of specific activities and kinetic constants of biotinidase and lipoamidase in LEW rat and ...
|
|
41459
|
Determination of specific activities and kinetic constants of biotinidase and lipoamidase in LEW rat and ...
|
|
41460
|
Determination of specific activities and kinetic constants of biotinidase and lipoamidase in LEW rat and ...
|
|
41461
|
Determination of specific activities and kinetic constants of biotinidase and lipoamidase in LEW rat and ...
|
|
41462
|
Determination of specific activities and kinetic constants of biotinidase and lipoamidase in LEW rat and ...
|
|
41463
|
Determination of specific activities and kinetic constants of biotinidase and lipoamidase in LEW rat and ...
|
|
41464
|
Determination of specific activities and kinetic constants of biotinidase and lipoamidase in LEW rat and ...
|
|
41465
|
Determination of specific activities and kinetic constants of biotinidase and lipoamidase in LEW rat and ...
|
|
41466
|
Determination of specific activities and kinetic constants of biotinidase and lipoamidase in LEW rat and ...
|
|
41467
|
Determination of specific activities and kinetic constants of biotinidase and lipoamidase in LEW rat and ...
|
|
41468
|
Determination of specific activities and kinetic constants of biotinidase and lipoamidase in LEW rat and ...
|
|
41469
|
Determination of specific activities and kinetic constants of biotinidase and lipoamidase in LEW rat and ...
|
|
41470
|
Determination of specific activities and kinetic constants of biotinidase and lipoamidase in LEW rat and ...
|
|
41471
|
Determination of specific activities and kinetic constants of biotinidase and lipoamidase in LEW rat and ...
|
|
41472
|
Determination of specific activities and kinetic constants of biotinidase and lipoamidase in LEW rat and ...
|
|
41473
|
Determination of specific activities and kinetic constants of biotinidase and lipoamidase in LEW rat and ...
|
|
41474
|
Determination of specific activities and kinetic constants of biotinidase and lipoamidase in LEW rat and ...
|
|
41475
|
Determination of specific activities and kinetic constants of biotinidase and lipoamidase in LEW rat and ...
|
|
41476
|
Determination of specific activities and kinetic constants of biotinidase and lipoamidase in LEW rat and ...
|
|
41477
|
Determination of specific activities and kinetic constants of biotinidase and lipoamidase in LEW rat and ...
|
|
41478
|
Determination of specific activities and kinetic constants of biotinidase and lipoamidase in LEW rat and ...
|
|
41479
|
Determination of specific activities and kinetic constants of biotinidase and lipoamidase in LEW rat and ...
|
|
41480
|
Determination of specific activities and kinetic constants of biotinidase and lipoamidase in LEW rat and ...
|
|
41481
|
Determination of specific activities and kinetic constants of biotinidase and lipoamidase in LEW rat and ...
|
|
41482
|
Determination of specific activities and kinetic constants of biotinidase and lipoamidase in LEW rat and ...
|
|
41483
|
Determination of specific activities and kinetic constants of biotinidase and lipoamidase in LEW rat and ...
|
|
41484
|
Determination of specific activities and kinetic constants of biotinidase and lipoamidase in LEW rat and ...
|
|
41485
|
Determination of specific activities and kinetic constants of biotinidase and lipoamidase in LEW rat and ...
|
|
41486
|
Determination of specific activities and kinetic constants of biotinidase and lipoamidase in LEW rat and ...
|
|
41487
|
Determination of specific activities and kinetic constants of biotinidase and lipoamidase in LEW rat and ...
|
|
41488
|
Determination of specific activities and kinetic constants of biotinidase and lipoamidase in LEW rat and ...
|
|
41489
|
Determination of specific activities and kinetic constants of biotinidase and lipoamidase in LEW rat and ...
|
|
41490
|
Determination of specific activities and kinetic constants of biotinidase and lipoamidase in LEW rat and ...
|
|
41491
|
Determination of specific activities and kinetic constants of biotinidase and lipoamidase in LEW rat and ...
|
|
41492
|
Determination of specific activities and kinetic constants of biotinidase and lipoamidase in LEW rat and ...
|
|
41493
|
Determination of specific activities and kinetic constants of biotinidase and lipoamidase in LEW rat and ...
|
|
41494
|
Determination of specific activities and kinetic constants of biotinidase and lipoamidase in LEW rat and ...
|
|
41495
|
Determination of specific activities and kinetic constants of biotinidase and lipoamidase in LEW rat and ...
|
|
41496
|
Determination of specific activities and kinetic constants of biotinidase and lipoamidase in LEW rat and ...
|
|
41497
|
Determination of specific activities and kinetic constants of biotinidase and lipoamidase in LEW rat and ...
|
|
41498
|
Determination of specific activities and kinetic constants of biotinidase and lipoamidase in LEW rat and ...
|
|
41499
|
Determination of specific activities and kinetic constants of biotinidase and lipoamidase in LEW rat and ...
|
|
41500
|
Determination of specific activities and kinetic constants of biotinidase and lipoamidase in LEW rat and ...
|
|
41501
|
Determination of specific activities and kinetic constants of biotinidase and lipoamidase in LEW rat and ...
|
|
41502
|
Determination of specific activities and kinetic constants of biotinidase and lipoamidase in LEW rat and ...
|
|
41503
|
Determination of specific activities and kinetic constants of biotinidase and lipoamidase in LEW rat and ...
|
|
41504
|
Determination of specific activities and kinetic constants of biotinidase and lipoamidase in LEW rat and ...
|
|
41505
|
Determination of specific activities and kinetic constants of biotinidase and lipoamidase in LEW rat and ...
|
|
41506
|
Determination of specific activities and kinetic constants of biotinidase and lipoamidase in LEW rat and ...
|
|
41507
|
Determination of specific activities and kinetic constants of biotinidase and lipoamidase in LEW rat and ...
|
|
41508
|
Determination of specific activities and kinetic constants of biotinidase and lipoamidase in LEW rat and ...
|
|
41509
|
Determination of specific activities and kinetic constants of biotinidase and lipoamidase in LEW rat and ...
|
|
41510
|
Determination of specific activities and kinetic constants of biotinidase and lipoamidase in LEW rat and ...
|
|
41511
|
Determination of specific activities and kinetic constants of biotinidase and lipoamidase in LEW rat and ...
|
|
41512
|
Determination of specific activities and kinetic constants of biotinidase and lipoamidase in LEW rat and ...
|
|
41513
|
Determination of specific activities and kinetic constants of biotinidase and lipoamidase in LEW rat and ...
|
|
41514
|
Determination of specific activities and kinetic constants of biotinidase and lipoamidase in LEW rat and ...
|
|
41515
|
Determination of specific activities and kinetic constants of biotinidase and lipoamidase in LEW rat and ...
|
|
41516
|
Determination of specific activities and kinetic constants of biotinidase and lipoamidase in LEW rat and ...
|
|
41517
|
Determination of specific activities and kinetic constants of biotinidase and lipoamidase in LEW rat and ...
|
|
41518
|
Determination of specific activities and kinetic constants of biotinidase and lipoamidase in LEW rat and ...
|
|
41519
|
Determination of specific activities and kinetic constants of biotinidase and lipoamidase in LEW rat and ...
|
|
41520
|
Determination of specific activities and kinetic constants of biotinidase and lipoamidase in LEW rat and ...
|
|
41521
|
Determination of specific activities and kinetic constants of biotinidase and lipoamidase in LEW rat and ...
|
|
41522
|
Determination of specific activities and kinetic constants of biotinidase and lipoamidase in LEW rat and ...
|
|
41523
|
Determination of specific activities and kinetic constants of biotinidase and lipoamidase in LEW rat and ...
|
|
41524
|
Determination of specific activities and kinetic constants of biotinidase and lipoamidase in LEW rat and ...
|
|
41525
|
Determination of specific activities and kinetic constants of biotinidase and lipoamidase in LEW rat and ...
|
|
41526
|
Determination of specific activities and kinetic constants of biotinidase and lipoamidase in LEW rat and ...
|
|
41527
|
Determination of specific activities and kinetic constants of biotinidase and lipoamidase in LEW rat and ...
|
|
41528
|
Determination of specific activities and kinetic constants of biotinidase and lipoamidase in LEW rat and ...
|
|
41529
|
Determination of specific activities and kinetic constants of biotinidase and lipoamidase in LEW rat and ...
|
|
41530
|
Determination of specific activities and kinetic constants of biotinidase and lipoamidase in LEW rat and ...
|
|
41531
|
Determination of specific activities and kinetic constants of biotinidase and lipoamidase in LEW rat and ...
|
|
41532
|
Determination of specific activities and kinetic constants of biotinidase and lipoamidase in LEW rat and ...
|
|
41533
|
Determination of specific activities and kinetic constants of biotinidase and lipoamidase in LEW rat and ...
|
|
41534
|
Determination of specific activities and kinetic constants of biotinidase and lipoamidase in LEW rat and ...
|
|
41535
|
Determination of specific activities and kinetic constants of biotinidase and lipoamidase in LEW rat and ...
|
|
41536
|
Determination of specific activities and kinetic constants of biotinidase and lipoamidase in LEW rat and ...
|
|
41537
|
Determination of specific activities and kinetic constants of biotinidase and lipoamidase in LEW rat and ...
|
|
41538
|
Determination of specific activities and kinetic constants of biotinidase and lipoamidase in LEW rat and ...
|
|
41539
|
Determination of specific activities and kinetic constants of biotinidase and lipoamidase in LEW rat and ...
|
|
41540
|
Determination of specific activities and kinetic constants of biotinidase and lipoamidase in LEW rat and ...
|
|
41541
|
Determination of specific activities and kinetic constants of biotinidase and lipoamidase in LEW rat and ...
|
|
41542
|
Determination of specific activities and kinetic constants of biotinidase and lipoamidase in LEW rat and ...
|
|
41543
|
Determination of specific activities and kinetic constants of biotinidase and lipoamidase in LEW rat and ...
|
|
41544
|
Determination of specific activities and kinetic constants of biotinidase and lipoamidase in LEW rat and ...
|
|
41545
|
Determination of specific activities and kinetic constants of biotinidase and lipoamidase in LEW rat and ...
|
|
41546
|
Determination of specific activities and kinetic constants of biotinidase and lipoamidase in LEW rat and ...
|
|
41547
|
Determination of specific activities and kinetic constants of biotinidase and lipoamidase in LEW rat and ...
|
|
41548
|
Determination of specific activities and kinetic constants of biotinidase and lipoamidase in LEW rat and ...
|
|
41549
|
Determination of specific activities and kinetic constants of biotinidase and lipoamidase in LEW rat and ...
|
|
41550
|
Determination of specific activities and kinetic constants of biotinidase and lipoamidase in LEW rat and ...
|
|
41551
|
Determination of specific activities and kinetic constants of biotinidase and lipoamidase in LEW rat and ...
|
|
41552
|
Determination of specific activities and kinetic constants of biotinidase and lipoamidase in LEW rat and ...
|
|
41553
|
Determination of specific activities and kinetic constants of biotinidase and lipoamidase in LEW rat and ...
|
|
41554
|
Determination of specific activities and kinetic constants of biotinidase and lipoamidase in LEW rat and ...
|
|
41555
|
Determination of specific activities and kinetic constants of biotinidase and lipoamidase in LEW rat and ...
|
|
41556
|
Determination of specific activities and kinetic constants of biotinidase and lipoamidase in LEW rat and ...
|
|
41557
|
Determination of specific activities and kinetic constants of biotinidase and lipoamidase in LEW rat and ...
|
|
41558
|
Determination of specific activities and kinetic constants of biotinidase and lipoamidase in LEW rat and ...
|
|
41559
|
Determination of specific activities and kinetic constants of biotinidase and lipoamidase in LEW rat and ...
|
|
41560
|
Determination of specific activities and kinetic constants of biotinidase and lipoamidase in LEW rat and ...
|
|
41561
|
Determination of specific activities and kinetic constants of biotinidase and lipoamidase in LEW rat and ...
|
|
41562
|
Determination of specific activities and kinetic constants of biotinidase and lipoamidase in LEW rat and ...
|
|
41563
|
Determination of specific activities and kinetic constants of biotinidase and lipoamidase in LEW rat and ...
|
|
41564
|
Determination of specific activities and kinetic constants of biotinidase and lipoamidase in LEW rat and ...
|
|
41565
|
Determination of specific activities and kinetic constants of biotinidase and lipoamidase in LEW rat and ...
|
|
41566
|
Determination of specific activities and kinetic constants of biotinidase and lipoamidase in LEW rat and ...
|
|
41567
|
Determination of specific activities and kinetic constants of biotinidase and lipoamidase in LEW rat and ...
|
|
41568
|
Determination of specific activities and kinetic constants of biotinidase and lipoamidase in LEW rat and ...
|
|
41569
|
Determination of specific activities and kinetic constants of biotinidase and lipoamidase in LEW rat and ...
|
|
41570
|
Determination of specific activities and kinetic constants of biotinidase and lipoamidase in LEW rat and ...
|
|
41571
|
Determination of specific activities and kinetic constants of biotinidase and lipoamidase in LEW rat and ...
|
|
41572
|
Determination of specific activities and kinetic constants of biotinidase and lipoamidase in LEW rat and ...
|
|
41573
|
Determination of specific activities and kinetic constants of biotinidase and lipoamidase in LEW rat and ...
|
|
41574
|
Determination of specific activities and kinetic constants of biotinidase and lipoamidase in LEW rat and ...
|
|
41575
|
Determination of specific activities and kinetic constants of biotinidase and lipoamidase in LEW rat and ...
|
|
41576
|
Determination of specific activities and kinetic constants of biotinidase and lipoamidase in LEW rat and ...
|
|
41577
|
Determination of specific activities and kinetic constants of biotinidase and lipoamidase in LEW rat and ...
|
|
41578
|
Determination of specific activities and kinetic constants of biotinidase and lipoamidase in LEW rat and ...
|
|
41579
|
Determination of specific activities and kinetic constants of biotinidase and lipoamidase in LEW rat and ...
|
|
41580
|
Determination of specific activities and kinetic constants of biotinidase and lipoamidase in LEW rat and ...
|
|
41581
|
Determination of specific activities and kinetic constants of biotinidase and lipoamidase in LEW rat and ...
|
|
41582
|
Determination of specific activities and kinetic constants of biotinidase and lipoamidase in LEW rat and ...
|
|
41583
|
Determination of specific activities and kinetic constants of biotinidase and lipoamidase in LEW rat and ...
|
|
41584
|
Determination of specific activities and kinetic constants of biotinidase and lipoamidase in LEW rat and ...
|
|
41585
|
Determination of specific activities and kinetic constants of biotinidase and lipoamidase in LEW rat and ...
|
|
41586
|
Determination of specific activities and kinetic constants of biotinidase and lipoamidase in LEW rat and ...
|
|
41587
|
Determination of specific activities and kinetic constants of biotinidase and lipoamidase in LEW rat and ...
|
|
41588
|
Determination of specific activities and kinetic constants of biotinidase and lipoamidase in LEW rat and ...
|
|
41589
|
Engineered Monomeric Human Histidine Triad Nucleotide-binding Protein 1 Hydrolyzes Fluorogenic Acyl-adenylate ...
|
|
41590
|
Engineered Monomeric Human Histidine Triad Nucleotide-binding Protein 1 Hydrolyzes Fluorogenic Acyl-adenylate ...
|
|
41591
|
Engineered Monomeric Human Histidine Triad Nucleotide-binding Protein 1 Hydrolyzes Fluorogenic Acyl-adenylate ...
|
|
41592
|
Engineered Monomeric Human Histidine Triad Nucleotide-binding Protein 1 Hydrolyzes Fluorogenic Acyl-adenylate ...
|
|
41593
|
Engineered Monomeric Human Histidine Triad Nucleotide-binding Protein 1 Hydrolyzes Fluorogenic Acyl-adenylate ...
|
|
41594
|
Engineered Monomeric Human Histidine Triad Nucleotide-binding Protein 1 Hydrolyzes Fluorogenic Acyl-adenylate ...
|
|
41595
|
Engineered Monomeric Human Histidine Triad Nucleotide-binding Protein 1 Hydrolyzes Fluorogenic Acyl-adenylate ...
|
|
41596
|
Engineered Monomeric Human Histidine Triad Nucleotide-binding Protein 1 Hydrolyzes Fluorogenic Acyl-adenylate ...
|
|
41597
|
Engineered Monomeric Human Histidine Triad Nucleotide-binding Protein 1 Hydrolyzes Fluorogenic Acyl-adenylate ...
|
|
41598
|
Engineered Monomeric Human Histidine Triad Nucleotide-binding Protein 1 Hydrolyzes Fluorogenic Acyl-adenylate ...
|
|
41599
|
Phosphoramidate pronucleotides: a comparison of the phosphoramidase substrate specificity of human and ...
|
|
41600
|
Phosphoramidate pronucleotides: a comparison of the phosphoramidase substrate specificity of human and ...
|
|
41601
|
Phosphoramidate pronucleotides: a comparison of the phosphoramidase substrate specificity of human and ...
|
|
41602
|
Phosphoramidate pronucleotides: a comparison of the phosphoramidase substrate specificity of human and ...
|
|
41603
|
Phosphoramidate pronucleotides: a comparison of the phosphoramidase substrate specificity of human and ...
|
|
41604
|
Phosphoramidate pronucleotides: a comparison of the phosphoramidase substrate specificity of human and ...
|
|
41605
|
Phosphoramidate pronucleotides: a comparison of the phosphoramidase substrate specificity of human and ...
|
|
41606
|
Phosphoramidate pronucleotides: a comparison of the phosphoramidase substrate specificity of human and ...
|
|
41607
|
Phosphoramidate pronucleotides: a comparison of the phosphoramidase substrate specificity of human and ...
|
|
41608
|
Phosphoramidate pronucleotides: a comparison of the phosphoramidase substrate specificity of human and ...
|
|
41609
|
Phosphoramidate pronucleotides: a comparison of the phosphoramidase substrate specificity of human and ...
|
|
41610
|
Phosphoramidate pronucleotides: a comparison of the phosphoramidase substrate specificity of human and ...
|
|
41611
|
Phosphoramidate pronucleotides: a comparison of the phosphoramidase substrate specificity of human and ...
|
|
41612
|
Phosphoramidate pronucleotides: a comparison of the phosphoramidase substrate specificity of human and ...
|
|
41613
|
Phosphoramidate pronucleotides: a comparison of the phosphoramidase substrate specificity of human and ...
|
|
41614
|
Phosphoramidate pronucleotides: a comparison of the phosphoramidase substrate specificity of human and ...
|
|
41615
|
Phosphoramidate pronucleotides: a comparison of the phosphoramidase substrate specificity of human and ...
|
|
41616
|
Phosphoramidate pronucleotides: a comparison of the phosphoramidase substrate specificity of human and ...
|
|
41617
|
Phosphoramidate pronucleotides: a comparison of the phosphoramidase substrate specificity of human and ...
|
|
41618
|
Phosphoramidate pronucleotides: a comparison of the phosphoramidase substrate specificity of human and ...
|
|
41619
|
Phosphoramidate pronucleotides: a comparison of the phosphoramidase substrate specificity of human and ...
|
|
41620
|
Phosphoramidate pronucleotides: a comparison of the phosphoramidase substrate specificity of human and ...
|
|
41621
|
Phosphoramidate pronucleotides: a comparison of the phosphoramidase substrate specificity of human and ...
|
|
41622
|
Phosphoramidate pronucleotides: a comparison of the phosphoramidase substrate specificity of human and ...
|
|
41623
|
Phosphoramidate pronucleotides: a comparison of the phosphoramidase substrate specificity of human and ...
|
|
41624
|
Phosphoramidate pronucleotides: a comparison of the phosphoramidase substrate specificity of human and ...
|
|
41625
|
Phosphoramidate pronucleotides: a comparison of the phosphoramidase substrate specificity of human and ...
|
|
41626
|
Phosphoramidate pronucleotides: a comparison of the phosphoramidase substrate specificity of human and ...
|
|
41627
|
Phosphoramidate pronucleotides: a comparison of the phosphoramidase substrate specificity of human and ...
|
|
41628
|
Phosphoramidate pronucleotides: a comparison of the phosphoramidase substrate specificity of human and ...
|
|
41629
|
Phosphoramidate pronucleotides: a comparison of the phosphoramidase substrate specificity of human and ...
|
|
41630
|
Phosphoramidate pronucleotides: a comparison of the phosphoramidase substrate specificity of human and ...
|
|
41631
|
Phosphoramidate pronucleotides: a comparison of the phosphoramidase substrate specificity of human and ...
|
|
41632
|
Phosphoramidate pronucleotides: a comparison of the phosphoramidase substrate specificity of human and ...
|
|
41633
|
Phosphoramidate pronucleotides: a comparison of the phosphoramidase substrate specificity of human and ...
|
|
41634
|
Phosphoramidate pronucleotides: a comparison of the phosphoramidase substrate specificity of human and ...
|
|
41635
|
Phosphoramidate pronucleotides: a comparison of the phosphoramidase substrate specificity of human and ...
|
|
41636
|
Phosphoramidate pronucleotides: a comparison of the phosphoramidase substrate specificity of human and ...
|
|
41637
|
Phosphoramidate pronucleotides: a comparison of the phosphoramidase substrate specificity of human and ...
|
|
41638
|
Phosphoramidate pronucleotides: a comparison of the phosphoramidase substrate specificity of human and ...
|
|
41639
|
Mouse 17alpha-hydroxysteroid dehydrogenase (AKR1C21) binds steroids differently from other aldo-keto ...
|
|
41640
|
Mouse 17alpha-hydroxysteroid dehydrogenase (AKR1C21) binds steroids differently from other aldo-keto ...
|
|
41641
|
Mouse 17alpha-hydroxysteroid dehydrogenase (AKR1C21) binds steroids differently from other aldo-keto ...
|
|
41642
|
Mouse 17alpha-hydroxysteroid dehydrogenase (AKR1C21) binds steroids differently from other aldo-keto ...
|
|
41643
|
Mouse 17alpha-hydroxysteroid dehydrogenase (AKR1C21) binds steroids differently from other aldo-keto ...
|
|
41644
|
Mouse 17alpha-hydroxysteroid dehydrogenase (AKR1C21) binds steroids differently from other aldo-keto ...
|
|
41645
|
Methionine regeneration and aminotransferases in Bacillus subtilis, Bacillus cereus, and Bacillus anthracis
|
|
41646
|
Methionine regeneration and aminotransferases in Bacillus subtilis, Bacillus cereus, and Bacillus anthracis
|
|
41647
|
Methionine regeneration and aminotransferases in Bacillus subtilis, Bacillus cereus, and Bacillus anthracis
|
|
41648
|
Methionine regeneration and aminotransferases in Bacillus subtilis, Bacillus cereus, and Bacillus anthracis
|
|
41649
|
Methionine regeneration and aminotransferases in Bacillus subtilis, Bacillus cereus, and Bacillus anthracis
|
|
41650
|
Methionine regeneration and aminotransferases in Bacillus subtilis, Bacillus cereus, and Bacillus anthracis
|
|
41651
|
Methionine regeneration and aminotransferases in Bacillus subtilis, Bacillus cereus, and Bacillus anthracis
|
|
41652
|
Methionine regeneration and aminotransferases in Bacillus subtilis, Bacillus cereus, and Bacillus anthracis
|
|
41653
|
Methionine regeneration and aminotransferases in Bacillus subtilis, Bacillus cereus, and Bacillus anthracis
|
|
41654
|
Methionine regeneration and aminotransferases in Bacillus subtilis, Bacillus cereus, and Bacillus anthracis
|
|
41655
|
Methionine regeneration and aminotransferases in Bacillus subtilis, Bacillus cereus, and Bacillus anthracis
|
|
41656
|
Methionine regeneration and aminotransferases in Bacillus subtilis, Bacillus cereus, and Bacillus anthracis
|
|
41657
|
Methionine regeneration and aminotransferases in Bacillus subtilis, Bacillus cereus, and Bacillus anthracis
|
|
41658
|
Methionine regeneration and aminotransferases in Bacillus subtilis, Bacillus cereus, and Bacillus anthracis
|
|
41659
|
Methionine regeneration and aminotransferases in Bacillus subtilis, Bacillus cereus, and Bacillus anthracis
|
|
41660
|
Methionine regeneration and aminotransferases in Bacillus subtilis, Bacillus cereus, and Bacillus anthracis
|
|
41661
|
Methionine regeneration and aminotransferases in Bacillus subtilis, Bacillus cereus, and Bacillus anthracis
|
|
41662
|
Methionine regeneration and aminotransferases in Bacillus subtilis, Bacillus cereus, and Bacillus anthracis
|
|
41663
|
Methionine regeneration and aminotransferases in Bacillus subtilis, Bacillus cereus, and Bacillus anthracis
|
|
41664
|
Methionine regeneration and aminotransferases in Bacillus subtilis, Bacillus cereus, and Bacillus anthracis
|
|
41665
|
Methionine regeneration and aminotransferases in Bacillus subtilis, Bacillus cereus, and Bacillus anthracis
|
|
41666
|
Methionine regeneration and aminotransferases in Bacillus subtilis, Bacillus cereus, and Bacillus anthracis
|
|
41667
|
Methionine regeneration and aminotransferases in Bacillus subtilis, Bacillus cereus, and Bacillus anthracis
|
|
41668
|
Methionine regeneration and aminotransferases in Bacillus subtilis, Bacillus cereus, and Bacillus anthracis
|
|
41669
|
Methionine regeneration and aminotransferases in Bacillus subtilis, Bacillus cereus, and Bacillus anthracis
|
|
41670
|
Methionine regeneration and aminotransferases in Bacillus subtilis, Bacillus cereus, and Bacillus anthracis
|
|
41671
|
Methionine regeneration and aminotransferases in Bacillus subtilis, Bacillus cereus, and Bacillus anthracis
|
|
41672
|
Methionine regeneration and aminotransferases in Bacillus subtilis, Bacillus cereus, and Bacillus anthracis
|
|
41673
|
Many fructosamine 3-kinase homologues in bacteria are ribulosamine/erythrulosamine 3-kinases potentially ...
|
|
41674
|
Many fructosamine 3-kinase homologues in bacteria are ribulosamine/erythrulosamine 3-kinases potentially ...
|
|
41675
|
Many fructosamine 3-kinase homologues in bacteria are ribulosamine/erythrulosamine 3-kinases potentially ...
|
|
41676
|
Many fructosamine 3-kinase homologues in bacteria are ribulosamine/erythrulosamine 3-kinases potentially ...
|
|
41677
|
Many fructosamine 3-kinase homologues in bacteria are ribulosamine/erythrulosamine 3-kinases potentially ...
|
|
41678
|
Many fructosamine 3-kinase homologues in bacteria are ribulosamine/erythrulosamine 3-kinases potentially ...
|
|
41679
|
Many fructosamine 3-kinase homologues in bacteria are ribulosamine/erythrulosamine 3-kinases potentially ...
|
|
41680
|
Many fructosamine 3-kinase homologues in bacteria are ribulosamine/erythrulosamine 3-kinases potentially ...
|
|
41681
|
Many fructosamine 3-kinase homologues in bacteria are ribulosamine/erythrulosamine 3-kinases potentially ...
|
|
41682
|
Many fructosamine 3-kinase homologues in bacteria are ribulosamine/erythrulosamine 3-kinases potentially ...
|
|
41683
|
Many fructosamine 3-kinase homologues in bacteria are ribulosamine/erythrulosamine 3-kinases potentially ...
|
|
41684
|
Many fructosamine 3-kinase homologues in bacteria are ribulosamine/erythrulosamine 3-kinases potentially ...
|
|
41685
|
Many fructosamine 3-kinase homologues in bacteria are ribulosamine/erythrulosamine 3-kinases potentially ...
|
|
41686
|
Many fructosamine 3-kinase homologues in bacteria are ribulosamine/erythrulosamine 3-kinases potentially ...
|
|
41687
|
Many fructosamine 3-kinase homologues in bacteria are ribulosamine/erythrulosamine 3-kinases potentially ...
|
|
41688
|
Many fructosamine 3-kinase homologues in bacteria are ribulosamine/erythrulosamine 3-kinases potentially ...
|
|
41689
|
Many fructosamine 3-kinase homologues in bacteria are ribulosamine/erythrulosamine 3-kinases potentially ...
|
|
41690
|
Many fructosamine 3-kinase homologues in bacteria are ribulosamine/erythrulosamine 3-kinases potentially ...
|
|
41691
|
Many fructosamine 3-kinase homologues in bacteria are ribulosamine/erythrulosamine 3-kinases potentially ...
|
|
41692
|
Many fructosamine 3-kinase homologues in bacteria are ribulosamine/erythrulosamine 3-kinases potentially ...
|
|
41693
|
Many fructosamine 3-kinase homologues in bacteria are ribulosamine/erythrulosamine 3-kinases potentially ...
|
|
41694
|
Many fructosamine 3-kinase homologues in bacteria are ribulosamine/erythrulosamine 3-kinases potentially ...
|
|
41695
|
Many fructosamine 3-kinase homologues in bacteria are ribulosamine/erythrulosamine 3-kinases potentially ...
|
|
41696
|
Many fructosamine 3-kinase homologues in bacteria are ribulosamine/erythrulosamine 3-kinases potentially ...
|
|
41697
|
Coenzyme A-disulfide reductase from Staphylococcus aureus: evidence for asymmetric behavior on interaction ...
|
|
41698
|
Coenzyme A-disulfide reductase from Staphylococcus aureus: evidence for asymmetric behavior on interaction ...
|
|
41699
|
Bisphosphonates target multiple sites in both cis- and trans-prenyltransferases
|
|
41700
|
Bisphosphonates target multiple sites in both cis- and trans-prenyltransferases
|
|
41701
|
Bisphosphonates target multiple sites in both cis- and trans-prenyltransferases
|
|
41702
|
Bisphosphonates target multiple sites in both cis- and trans-prenyltransferases
|
|
41703
|
Bisphosphonates target multiple sites in both cis- and trans-prenyltransferases
|
|
41704
|
Bisphosphonates target multiple sites in both cis- and trans-prenyltransferases
|
|
41705
|
Bisphosphonates target multiple sites in both cis- and trans-prenyltransferases
|
|
41706
|
Bisphosphonates target multiple sites in both cis- and trans-prenyltransferases
|
|
41707
|
Bisphosphonates target multiple sites in both cis- and trans-prenyltransferases
|
|
41708
|
Bisphosphonates target multiple sites in both cis- and trans-prenyltransferases
|
|
41709
|
Bisphosphonates target multiple sites in both cis- and trans-prenyltransferases
|
|
41710
|
Bisphosphonates target multiple sites in both cis- and trans-prenyltransferases
|
|
41711
|
Bisphosphonates target multiple sites in both cis- and trans-prenyltransferases
|
|
41712
|
Bisphosphonates target multiple sites in both cis- and trans-prenyltransferases
|
|
41713
|
Bisphosphonates target multiple sites in both cis- and trans-prenyltransferases
|
|
41714
|
Bisphosphonates target multiple sites in both cis- and trans-prenyltransferases
|
|
41715
|
Bisphosphonates target multiple sites in both cis- and trans-prenyltransferases
|
|
41716
|
Bisphosphonates target multiple sites in both cis- and trans-prenyltransferases
|
|
41717
|
Common-type acylphosphatase: steady-state kinetics and leaving-group dependence
|
|
41718
|
Common-type acylphosphatase: steady-state kinetics and leaving-group dependence
|
|
41719
|
Common-type acylphosphatase: steady-state kinetics and leaving-group dependence
|
|
41720
|
Common-type acylphosphatase: steady-state kinetics and leaving-group dependence
|
|
41721
|
Common-type acylphosphatase: steady-state kinetics and leaving-group dependence
|
|
41722
|
Common-type acylphosphatase: steady-state kinetics and leaving-group dependence
|
|
41723
|
Common-type acylphosphatase: steady-state kinetics and leaving-group dependence
|
|
41724
|
Common-type acylphosphatase: steady-state kinetics and leaving-group dependence
|
|
41725
|
A novel beta-galactosidase capable of glycosyl transfer from Enterobacter agglomerans B1
|
|
41726
|
A novel beta-galactosidase capable of glycosyl transfer from Enterobacter agglomerans B1
|
|
41727
|
Escherichia coli glyoxalase II is a binuclear zinc-dependent metalloenzyme
|
|
41728
|
Diverging Substrate Specificity of Pure Human Thymidine Kinases 1 and 2 Against Antiviral Dideoxynucleosides
|
|
41729
|
Diverging Substrate Specificity of Pure Human Thymidine Kinases 1 and 2 Against Antiviral Dideoxynucleosides
|
|
41730
|
Diverging Substrate Specificity of Pure Human Thymidine Kinases 1 and 2 Against Antiviral Dideoxynucleosides
|
|
41731
|
Diverging Substrate Specificity of Pure Human Thymidine Kinases 1 and 2 Against Antiviral Dideoxynucleosides
|
|
41732
|
Diverging Substrate Specificity of Pure Human Thymidine Kinases 1 and 2 Against Antiviral Dideoxynucleosides
|
|
41733
|
Diverging Substrate Specificity of Pure Human Thymidine Kinases 1 and 2 Against Antiviral Dideoxynucleosides
|
|
41734
|
Diverging Substrate Specificity of Pure Human Thymidine Kinases 1 and 2 Against Antiviral Dideoxynucleosides
|
|
41735
|
Diverging Substrate Specificity of Pure Human Thymidine Kinases 1 and 2 Against Antiviral Dideoxynucleosides
|
|
41736
|
Diverging Substrate Specificity of Pure Human Thymidine Kinases 1 and 2 Against Antiviral Dideoxynucleosides
|
|
41737
|
Diverging Substrate Specificity of Pure Human Thymidine Kinases 1 and 2 Against Antiviral Dideoxynucleosides
|
|
41738
|
Diverging Substrate Specificity of Pure Human Thymidine Kinases 1 and 2 Against Antiviral Dideoxynucleosides
|
|
41739
|
Diverging Substrate Specificity of Pure Human Thymidine Kinases 1 and 2 Against Antiviral Dideoxynucleosides
|
|
41740
|
Diverging Substrate Specificity of Pure Human Thymidine Kinases 1 and 2 Against Antiviral Dideoxynucleosides
|
|
41741
|
Diverging Substrate Specificity of Pure Human Thymidine Kinases 1 and 2 Against Antiviral Dideoxynucleosides
|
|
41742
|
Diverging Substrate Specificity of Pure Human Thymidine Kinases 1 and 2 Against Antiviral Dideoxynucleosides
|
|
41743
|
Diverging Substrate Specificity of Pure Human Thymidine Kinases 1 and 2 Against Antiviral Dideoxynucleosides
|
|
41744
|
Diverging Substrate Specificity of Pure Human Thymidine Kinases 1 and 2 Against Antiviral Dideoxynucleosides
|
|
41745
|
Diverging Substrate Specificity of Pure Human Thymidine Kinases 1 and 2 Against Antiviral Dideoxynucleosides
|
|
41746
|
Diverging Substrate Specificity of Pure Human Thymidine Kinases 1 and 2 Against Antiviral Dideoxynucleosides
|
|
41747
|
Diverging Substrate Specificity of Pure Human Thymidine Kinases 1 and 2 Against Antiviral Dideoxynucleosides
|
|
41748
|
Diverging Substrate Specificity of Pure Human Thymidine Kinases 1 and 2 Against Antiviral Dideoxynucleosides
|
|
41749
|
Diverging Substrate Specificity of Pure Human Thymidine Kinases 1 and 2 Against Antiviral Dideoxynucleosides
|
|
41750
|
Diverging Substrate Specificity of Pure Human Thymidine Kinases 1 and 2 Against Antiviral Dideoxynucleosides
|
|
41751
|
Diverging Substrate Specificity of Pure Human Thymidine Kinases 1 and 2 Against Antiviral Dideoxynucleosides
|
|
41752
|
Diverging Substrate Specificity of Pure Human Thymidine Kinases 1 and 2 Against Antiviral Dideoxynucleosides
|
|
41753
|
Diverging Substrate Specificity of Pure Human Thymidine Kinases 1 and 2 Against Antiviral Dideoxynucleosides
|
|
41754
|
Diverging Substrate Specificity of Pure Human Thymidine Kinases 1 and 2 Against Antiviral Dideoxynucleosides
|
|
41755
|
Diverging Substrate Specificity of Pure Human Thymidine Kinases 1 and 2 Against Antiviral Dideoxynucleosides
|
|
41756
|
Diverging Substrate Specificity of Pure Human Thymidine Kinases 1 and 2 Against Antiviral Dideoxynucleosides
|
|
41757
|
Diverging Substrate Specificity of Pure Human Thymidine Kinases 1 and 2 Against Antiviral Dideoxynucleosides
|
|
41758
|
Diverging Substrate Specificity of Pure Human Thymidine Kinases 1 and 2 Against Antiviral Dideoxynucleosides
|
|
41759
|
Diverging Substrate Specificity of Pure Human Thymidine Kinases 1 and 2 Against Antiviral Dideoxynucleosides
|
|
41760
|
Diverging Substrate Specificity of Pure Human Thymidine Kinases 1 and 2 Against Antiviral Dideoxynucleosides
|
|
41761
|
Diverging Substrate Specificity of Pure Human Thymidine Kinases 1 and 2 Against Antiviral Dideoxynucleosides
|
|
41762
|
Diverging Substrate Specificity of Pure Human Thymidine Kinases 1 and 2 Against Antiviral Dideoxynucleosides
|
|
41763
|
Diverging Substrate Specificity of Pure Human Thymidine Kinases 1 and 2 Against Antiviral Dideoxynucleosides
|
|
41764
|
Diverging Substrate Specificity of Pure Human Thymidine Kinases 1 and 2 Against Antiviral Dideoxynucleosides
|
|
41765
|
Diverging Substrate Specificity of Pure Human Thymidine Kinases 1 and 2 Against Antiviral Dideoxynucleosides
|
|
41766
|
Diverging Substrate Specificity of Pure Human Thymidine Kinases 1 and 2 Against Antiviral Dideoxynucleosides
|
|
41767
|
Diverging Substrate Specificity of Pure Human Thymidine Kinases 1 and 2 Against Antiviral Dideoxynucleosides
|
|
41768
|
Diverging Substrate Specificity of Pure Human Thymidine Kinases 1 and 2 Against Antiviral Dideoxynucleosides
|
|
41769
|
Structural basis for shikimate-binding specificity of Helicobacter pylori shikimate kinase
|
|
41770
|
Structural basis for shikimate-binding specificity of Helicobacter pylori shikimate kinase
|
|
41771
|
Nuclear export of the DEAD box An3 protein by CRM1 is coupled to An3 helicase activity
|
|
41772
|
Identification of coenzyme M biosynthetic phosphosulfolactate synthase: a new family of ...
|
|
41773
|
Identification of coenzyme M biosynthetic phosphosulfolactate synthase: a new family of ...
|
|
41774
|
Identification of coenzyme M biosynthetic phosphosulfolactate synthase: a new family of ...
|
|
41775
|
Identification of coenzyme M biosynthetic phosphosulfolactate synthase: a new family of ...
|
|
41776
|
Identification of coenzyme M biosynthetic phosphosulfolactate synthase: a new family of ...
|
|
41777
|
Identification of coenzyme M biosynthetic phosphosulfolactate synthase: a new family of ...
|
|
41778
|
An unusual isopentenyl diphosphate isomerase found in the mevalonate pathway gene cluster from Streptomyces ...
|
|
41779
|
An unusual isopentenyl diphosphate isomerase found in the mevalonate pathway gene cluster from Streptomyces ...
|
|
41780
|
Purification and properties of lactaldehyde dehydrogenase from Escherichia coli
|
|
41781
|
Purification and properties of lactaldehyde dehydrogenase from Escherichia coli
|
|
41782
|
Biochemical characterization of androgen receptor-interacting protein 4
|
|
41783
|
Biochemical characterization of androgen receptor-interacting protein 4
|
|
41784
|
Molecular characterization of dipeptidyl peptidase activity in serum: soluble CD26/dipeptidyl peptidase IV is ...
|
|
41785
|
Molecular characterization of dipeptidyl peptidase activity in serum: soluble CD26/dipeptidyl peptidase IV is ...
|
|
41786
|
Molecular characterization of dipeptidyl peptidase activity in serum: soluble CD26/dipeptidyl peptidase IV is ...
|
|
41787
|
Molecular characterization of dipeptidyl peptidase activity in serum: soluble CD26/dipeptidyl peptidase IV is ...
|
|
41788
|
Molecular characterization of dipeptidyl peptidase activity in serum: soluble CD26/dipeptidyl peptidase IV is ...
|
|
41789
|
Molecular characterization of dipeptidyl peptidase activity in serum: soluble CD26/dipeptidyl peptidase IV is ...
|
|
41790
|
Molecular characterization of dipeptidyl peptidase activity in serum: soluble CD26/dipeptidyl peptidase IV is ...
|
|
41791
|
Molecular characterization of dipeptidyl peptidase activity in serum: soluble CD26/dipeptidyl peptidase IV is ...
|
|
41792
|
Molecular characterization of dipeptidyl peptidase activity in serum: soluble CD26/dipeptidyl peptidase IV is ...
|
|
41793
|
Gene expression and characterization of a stress-induced tyrosine decarboxylase from Arabidopsis thaliana
|
|
41794
|
Continuous spectrophotometric assay of peptide deformylase
|
|
41795
|
Continuous spectrophotometric assay of peptide deformylase
|
|
41796
|
Continuous spectrophotometric assay of peptide deformylase
|
|
41797
|
Continuous spectrophotometric assay of peptide deformylase
|
|
41798
|
Continuous spectrophotometric assay of peptide deformylase
|
|
41799
|
Continuous spectrophotometric assay of peptide deformylase
|
|
41800
|
Some kinetic properties of Bacillus subtilis glutamine synthetase
|
|
41801
|
Some kinetic properties of Bacillus subtilis glutamine synthetase
|
|
41802
|
Some kinetic properties of Bacillus subtilis glutamine synthetase
|
|
41803
|
Characterization of a lysK gene as an argE homolog in Thermus thermophilus HB27
|
|
41804
|
Characterization of a lysK gene as an argE homolog in Thermus thermophilus HB27
|
|
41805
|
Purification and characterization of the first archaeal aconitase from the thermoacidophilic Sulfolobus ...
|
|
41806
|
Purification and characterization of the first archaeal aconitase from the thermoacidophilic Sulfolobus ...
|
|
41807
|
Purification and characterization of the first archaeal aconitase from the thermoacidophilic Sulfolobus ...
|
|
41808
|
Purification and characterization of the first archaeal aconitase from the thermoacidophilic Sulfolobus ...
|
|
41809
|
Purification and characterization of the first archaeal aconitase from the thermoacidophilic Sulfolobus ...
|
|
41810
|
Purification and characterization of the first archaeal aconitase from the thermoacidophilic Sulfolobus ...
|
|
41811
|
Purification and characterization of the first archaeal aconitase from the thermoacidophilic Sulfolobus ...
|
|
41812
|
Purification and characterization of the first archaeal aconitase from the thermoacidophilic Sulfolobus ...
|
|
41813
|
Kinetics and product analysis of the reaction catalysed by recombinant homoaconitase from Thermus thermophilus
|
|
41814
|
Kinetics and product analysis of the reaction catalysed by recombinant homoaconitase from Thermus thermophilus
|
|
41815
|
Identification and purification of glucose phosphate isomerase of Plasmodium falciparum
|
|
41816
|
Identification and purification of glucose phosphate isomerase of Plasmodium falciparum
|
|
41817
|
Identification and purification of glucose phosphate isomerase of Plasmodium falciparum
|
|
41818
|
Identification and purification of glucose phosphate isomerase of Plasmodium falciparum
|
|
41819
|
D-lactate dehydrogenase is a member of the D-isomer-specific 2-hydroxyacid dehydrogenase family. Cloning, ...
|
|
41820
|
D-lactate dehydrogenase is a member of the D-isomer-specific 2-hydroxyacid dehydrogenase family. Cloning, ...
|
|
41821
|
D-lactate dehydrogenase is a member of the D-isomer-specific 2-hydroxyacid dehydrogenase family. Cloning, ...
|
|
41822
|
D-lactate dehydrogenase is a member of the D-isomer-specific 2-hydroxyacid dehydrogenase family. Cloning, ...
|
|
41823
|
D-lactate dehydrogenase is a member of the D-isomer-specific 2-hydroxyacid dehydrogenase family. Cloning, ...
|
|
41824
|
D-lactate dehydrogenase is a member of the D-isomer-specific 2-hydroxyacid dehydrogenase family. Cloning, ...
|
|
41825
|
D-lactate dehydrogenase is a member of the D-isomer-specific 2-hydroxyacid dehydrogenase family. Cloning, ...
|
|
41826
|
D-lactate dehydrogenase is a member of the D-isomer-specific 2-hydroxyacid dehydrogenase family. Cloning, ...
|
|
41827
|
D-lactate dehydrogenase is a member of the D-isomer-specific 2-hydroxyacid dehydrogenase family. Cloning, ...
|
|
41828
|
Histidine 296 is essential for the catalysis in Lactobacillus plantarum D-lactate dehydrogenase
|
|
41829
|
Histidine 296 is essential for the catalysis in Lactobacillus plantarum D-lactate dehydrogenase
|
|
41830
|
Histidine 296 is essential for the catalysis in Lactobacillus plantarum D-lactate dehydrogenase
|
|
41831
|
Histidine 296 is essential for the catalysis in Lactobacillus plantarum D-lactate dehydrogenase
|
|
41832
|
Histidine 296 is essential for the catalysis in Lactobacillus plantarum D-lactate dehydrogenase
|
|
41833
|
An operon from Lactobacillus helveticus composed of a proline iminopeptidase gene (pepI) and two genes coding ...
|
|
41834
|
An h.p.l.c. assay for protoporphyrinogen oxidase activity in rat liver
|
|
41835
|
Kinetics and equilibrium of the inactivation of ornithine transcarbamylases by pyridoxal 5`-phosphate
|
|
41836
|
Kinetics and equilibrium of the inactivation of ornithine transcarbamylases by pyridoxal 5`-phosphate
|
|
41837
|
Kinetics and equilibrium of the inactivation of ornithine transcarbamylases by pyridoxal 5`-phosphate
|
|
41838
|
Kinetics and equilibrium of the inactivation of ornithine transcarbamylases by pyridoxal 5`-phosphate
|
|
41839
|
Kinetics and equilibrium of the inactivation of ornithine transcarbamylases by pyridoxal 5`-phosphate
|
|
41840
|
Kinetics and equilibrium of the inactivation of ornithine transcarbamylases by pyridoxal 5`-phosphate
|
|
41841
|
Kinetics and equilibrium of the inactivation of ornithine transcarbamylases by pyridoxal 5`-phosphate
|
|
41842
|
Kinetics and equilibrium of the inactivation of ornithine transcarbamylases by pyridoxal 5`-phosphate
|
|
41843
|
Kinetics and equilibrium of the inactivation of ornithine transcarbamylases by pyridoxal 5`-phosphate
|
|
41844
|
Kinetics and equilibrium of the inactivation of ornithine transcarbamylases by pyridoxal 5`-phosphate
|
|
41845
|
Kinetics and equilibrium of the inactivation of ornithine transcarbamylases by pyridoxal 5`-phosphate
|
|
41846
|
Kinetics and equilibrium of the inactivation of ornithine transcarbamylases by pyridoxal 5`-phosphate
|
|
41847
|
Kinetics and equilibrium of the inactivation of ornithine transcarbamylases by pyridoxal 5`-phosphate
|
|
41848
|
Kinetics and equilibrium of the inactivation of ornithine transcarbamylases by pyridoxal 5`-phosphate
|
|
41849
|
Kinetics and equilibrium of the inactivation of ornithine transcarbamylases by pyridoxal 5`-phosphate
|
|
41850
|
Kinetics and equilibrium of the inactivation of ornithine transcarbamylases by pyridoxal 5`-phosphate
|
|
41851
|
Kinetic and structural characterization of phosphofructokinase from Lactobacillus bulgaricus
|
|
41852
|
Kinetic and structural characterization of phosphofructokinase from Lactobacillus bulgaricus
|
|
41853
|
Kinetic and structural characterization of phosphofructokinase from Lactobacillus bulgaricus
|
|
41854
|
Kinetic and structural characterization of phosphofructokinase from Lactobacillus bulgaricus
|
|
41855
|
Kinetic and structural characterization of phosphofructokinase from Lactobacillus bulgaricus
|
|
41856
|
Phenylalanine ammonia-lyase (PAL) from tobacco (Nicotiana tabacum): characterization of the four tobacco PAL ...
|
|
41857
|
Phenylalanine ammonia-lyase (PAL) from tobacco (Nicotiana tabacum): characterization of the four tobacco PAL ...
|
|
41858
|
Phenylalanine ammonia-lyase (PAL) from tobacco (Nicotiana tabacum): characterization of the four tobacco PAL ...
|
|
41859
|
Phenylalanine ammonia-lyase (PAL) from tobacco (Nicotiana tabacum): characterization of the four tobacco PAL ...
|
|
41860
|
Phenylalanine ammonia-lyase (PAL) from tobacco (Nicotiana tabacum): characterization of the four tobacco PAL ...
|
|
41861
|
Phenylalanine ammonia-lyase (PAL) from tobacco (Nicotiana tabacum): characterization of the four tobacco PAL ...
|
|
41862
|
Phenylalanine ammonia-lyase (PAL) from tobacco (Nicotiana tabacum): characterization of the four tobacco PAL ...
|
|
41863
|
Phenylalanine ammonia-lyase (PAL) from tobacco (Nicotiana tabacum): characterization of the four tobacco PAL ...
|
|
41864
|
Phenylalanine ammonia-lyase (PAL) from tobacco (Nicotiana tabacum): characterization of the four tobacco PAL ...
|
|
41865
|
Alpha-amylase from germinating soybean (Glycine max) seeds--purification, characterization and sequential ...
|
|
41866
|
Alpha-amylase from germinating soybean (Glycine max) seeds--purification, characterization and sequential ...
|
|
41867
|
Beta-galactosidase (Escherichia coli) has a second catalytically important Mg2+ site
|
|
41868
|
Beta-galactosidase (Escherichia coli) has a second catalytically important Mg2+ site
|
|
41869
|
Beta-galactosidase (Escherichia coli) has a second catalytically important Mg2+ site
|
|
41870
|
Beta-galactosidase (Escherichia coli) has a second catalytically important Mg2+ site
|
|
41871
|
Beta-galactosidase (Escherichia coli) has a second catalytically important Mg2+ site
|
|
41872
|
Beta-galactosidase (Escherichia coli) has a second catalytically important Mg2+ site
|
|
41873
|
Beta-galactosidase (Escherichia coli) has a second catalytically important Mg2+ site
|
|
41874
|
Beta-galactosidase (Escherichia coli) has a second catalytically important Mg2+ site
|
|
41875
|
Beta-galactosidase (Escherichia coli) has a second catalytically important Mg2+ site
|
|
41876
|
Beta-galactosidase (Escherichia coli) has a second catalytically important Mg2+ site
|
|
41877
|
Beta-galactosidase (Escherichia coli) has a second catalytically important Mg2+ site
|
|
41878
|
Beta-galactosidase (Escherichia coli) has a second catalytically important Mg2+ site
|
|
41879
|
Beta-galactosidase (Escherichia coli) has a second catalytically important Mg2+ site
|
|
41880
|
Novel flavonol 3-sulfotransferase. Purification, kinetic properties, and partial amino acid sequence
|
|
41881
|
Novel flavonol 3-sulfotransferase. Purification, kinetic properties, and partial amino acid sequence
|
|
41882
|
Novel flavonol 3-sulfotransferase. Purification, kinetic properties, and partial amino acid sequence
|
|
41883
|
Novel flavonol 3-sulfotransferase. Purification, kinetic properties, and partial amino acid sequence
|
|
41884
|
Novel flavonol 3-sulfotransferase. Purification, kinetic properties, and partial amino acid sequence
|
|
41885
|
Novel flavonol 3-sulfotransferase. Purification, kinetic properties, and partial amino acid sequence
|
|
41886
|
The 2-oxoglutarate binding site of prolyl 4-hydroxylase. Identification of distinct subsites and evidence for ...
|
|
41887
|
The 2-oxoglutarate binding site of prolyl 4-hydroxylase. Identification of distinct subsites and evidence for ...
|
|
41888
|
The 2-oxoglutarate binding site of prolyl 4-hydroxylase. Identification of distinct subsites and evidence for ...
|
|
41889
|
The 2-oxoglutarate binding site of prolyl 4-hydroxylase. Identification of distinct subsites and evidence for ...
|
|
41890
|
The 2-oxoglutarate binding site of prolyl 4-hydroxylase. Identification of distinct subsites and evidence for ...
|
|
41891
|
The 2-oxoglutarate binding site of prolyl 4-hydroxylase. Identification of distinct subsites and evidence for ...
|
|
41892
|
The 2-oxoglutarate binding site of prolyl 4-hydroxylase. Identification of distinct subsites and evidence for ...
|
|
41893
|
The 2-oxoglutarate binding site of prolyl 4-hydroxylase. Identification of distinct subsites and evidence for ...
|
|
41894
|
The 2-oxoglutarate binding site of prolyl 4-hydroxylase. Identification of distinct subsites and evidence for ...
|
|
41895
|
The 2-oxoglutarate binding site of prolyl 4-hydroxylase. Identification of distinct subsites and evidence for ...
|
|
41896
|
The 2-oxoglutarate binding site of prolyl 4-hydroxylase. Identification of distinct subsites and evidence for ...
|
|
41897
|
The 2-oxoglutarate binding site of prolyl 4-hydroxylase. Identification of distinct subsites and evidence for ...
|
|
41898
|
The 2-oxoglutarate binding site of prolyl 4-hydroxylase. Identification of distinct subsites and evidence for ...
|
|
41899
|
The 2-oxoglutarate binding site of prolyl 4-hydroxylase. Identification of distinct subsites and evidence for ...
|
|
41900
|
The 2-oxoglutarate binding site of prolyl 4-hydroxylase. Identification of distinct subsites and evidence for ...
|
|
41901
|
The 2-oxoglutarate binding site of prolyl 4-hydroxylase. Identification of distinct subsites and evidence for ...
|
|
41902
|
The 2-oxoglutarate binding site of prolyl 4-hydroxylase. Identification of distinct subsites and evidence for ...
|
|
41903
|
The 2-oxoglutarate binding site of prolyl 4-hydroxylase. Identification of distinct subsites and evidence for ...
|
|
41904
|
The 2-oxoglutarate binding site of prolyl 4-hydroxylase. Identification of distinct subsites and evidence for ...
|
|
41905
|
The 2-oxoglutarate binding site of prolyl 4-hydroxylase. Identification of distinct subsites and evidence for ...
|
|
41906
|
The 2-oxoglutarate binding site of prolyl 4-hydroxylase. Identification of distinct subsites and evidence for ...
|
|
41907
|
The 2-oxoglutarate binding site of prolyl 4-hydroxylase. Identification of distinct subsites and evidence for ...
|
|
41908
|
The 2-oxoglutarate binding site of prolyl 4-hydroxylase. Identification of distinct subsites and evidence for ...
|
|
41909
|
Cytochrome P-450TYR is a multifunctional heme-thiolate enzyme catalyzing the conversion of L-tyrosine to ...
|
|
41910
|
Cytochrome P-450TYR is a multifunctional heme-thiolate enzyme catalyzing the conversion of L-tyrosine to ...
|
|
41911
|
Properties and reaction mechanism of C4 leaf pyruvate,Pi dikinase
|
|
41912
|
Properties and reaction mechanism of C4 leaf pyruvate,Pi dikinase
|
|
41913
|
Properties and reaction mechanism of C4 leaf pyruvate,Pi dikinase
|
|
41914
|
Properties and reaction mechanism of C4 leaf pyruvate,Pi dikinase
|
|
41915
|
Properties and reaction mechanism of C4 leaf pyruvate,Pi dikinase
|
|
41916
|
Properties and reaction mechanism of C4 leaf pyruvate,Pi dikinase
|
|
41917
|
Properties and reaction mechanism of C4 leaf pyruvate,Pi dikinase
|
|
41918
|
Properties and reaction mechanism of C4 leaf pyruvate,Pi dikinase
|
|
41919
|
Properties and reaction mechanism of C4 leaf pyruvate,Pi dikinase
|
|
41920
|
Properties and reaction mechanism of C4 leaf pyruvate,Pi dikinase
|
|
41921
|
Properties and reaction mechanism of C4 leaf pyruvate,Pi dikinase
|
|
41922
|
Properties and reaction mechanism of C4 leaf pyruvate,Pi dikinase
|
|
41923
|
Properties and reaction mechanism of C4 leaf pyruvate,Pi dikinase
|
|
41924
|
Properties and reaction mechanism of C4 leaf pyruvate,Pi dikinase
|
|
41925
|
Properties and reaction mechanism of C4 leaf pyruvate,Pi dikinase
|
|
41926
|
Properties and reaction mechanism of C4 leaf pyruvate,Pi dikinase
|
|
41927
|
Properties and reaction mechanism of C4 leaf pyruvate,Pi dikinase
|
|
41928
|
Properties and reaction mechanism of C4 leaf pyruvate,Pi dikinase
|
|
41929
|
Properties and reaction mechanism of C4 leaf pyruvate,Pi dikinase
|
|
41930
|
Identification of the amino acid residues responsible for cold tolerance in Flaveria brownii ...
|
|
41931
|
Identification of the amino acid residues responsible for cold tolerance in Flaveria brownii ...
|
|
41932
|
Identification of the amino acid residues responsible for cold tolerance in Flaveria brownii ...
|
|
41933
|
Identification of the amino acid residues responsible for cold tolerance in Flaveria brownii ...
|
|
41934
|
Identification of the amino acid residues responsible for cold tolerance in Flaveria brownii ...
|
|
41935
|
Identification of the amino acid residues responsible for cold tolerance in Flaveria brownii ...
|
|
41936
|
Structural requirements for catalysis, expression, and dimerization in the CD26/DPIV gene family
|
|
41937
|
Structural requirements for catalysis, expression, and dimerization in the CD26/DPIV gene family
|
|
41938
|
Mechanism of elementary catalytic steps of pyruvate oxidase from Lactobacillus plantarum
|
|
41939
|
Mechanism of elementary catalytic steps of pyruvate oxidase from Lactobacillus plantarum
|
|
41940
|
Cloning, expression and chromosomal localization of a novel human dipeptidyl peptidase (DPP) IV homolog, DPP8
|
|
41941
|
Cloning, expression and chromosomal localization of a novel human dipeptidyl peptidase (DPP) IV homolog, DPP8
|
|
41942
|
Cloning, expression and chromosomal localization of a novel human dipeptidyl peptidase (DPP) IV homolog, DPP8
|
|
41943
|
Cloning, expression and chromosomal localization of a novel human dipeptidyl peptidase (DPP) IV homolog, DPP8
|
|
41944
|
Oxygen exchange between acetate and the catalytic glutamate residue in glutaconate CoA-transferase from ...
|
|
41945
|
Oxygen exchange between acetate and the catalytic glutamate residue in glutaconate CoA-transferase from ...
|
|
41946
|
Expression of apple 1-aminocyclopropane-1-carboxylate synthase in Escherichia coli: kinetic characterization ...
|
|
41947
|
Expression of apple 1-aminocyclopropane-1-carboxylate synthase in Escherichia coli: kinetic characterization ...
|
|
41948
|
Expression of apple 1-aminocyclopropane-1-carboxylate synthase in Escherichia coli: kinetic characterization ...
|
|
41949
|
Pyridine nucleotide complexes with Bacillus anthracis coenzyme A-disulfide reductase: a structural analysis of ...
|
|
41950
|
Pyridine nucleotide complexes with Bacillus anthracis coenzyme A-disulfide reductase: a structural analysis of ...
|
|
41951
|
Pyridine nucleotide complexes with Bacillus anthracis coenzyme A-disulfide reductase: a structural analysis of ...
|
|
41952
|
Pyridine nucleotide complexes with Bacillus anthracis coenzyme A-disulfide reductase: a structural analysis of ...
|
|
41953
|
Pyridine nucleotide complexes with Bacillus anthracis coenzyme A-disulfide reductase: a structural analysis of ...
|
|
41954
|
Pyridine nucleotide complexes with Bacillus anthracis coenzyme A-disulfide reductase: a structural analysis of ...
|
|
41955
|
Bovine pyruvate kinases. II. Purification of the liver isozyme and its hybridization with skeletal muscle ...
|
|
41956
|
Bovine pyruvate kinases. II. Purification of the liver isozyme and its hybridization with skeletal muscle ...
|
|
41957
|
Bovine pyruvate kinases. II. Purification of the liver isozyme and its hybridization with skeletal muscle ...
|
|
41958
|
Bovine pyruvate kinases. II. Purification of the liver isozyme and its hybridization with skeletal muscle ...
|
|
41959
|
Mutational analysis of domain II of flavonol 3-sulfotransferase
|
|
41960
|
Mutational analysis of domain II of flavonol 3-sulfotransferase
|
|
41961
|
Mutational analysis of domain II of flavonol 3-sulfotransferase
|
|
41962
|
Mutational analysis of domain II of flavonol 3-sulfotransferase
|
|
41963
|
Mutational analysis of domain II of flavonol 3-sulfotransferase
|
|
41964
|
Mutational analysis of domain II of flavonol 3-sulfotransferase
|
|
41965
|
Mutational analysis of domain II of flavonol 3-sulfotransferase
|
|
41966
|
Mutational analysis of domain II of flavonol 3-sulfotransferase
|
|
41967
|
Mutational analysis of domain II of flavonol 3-sulfotransferase
|
|
41968
|
Mutational analysis of domain II of flavonol 3-sulfotransferase
|
|
41969
|
Mutational analysis of domain II of flavonol 3-sulfotransferase
|
|
41970
|
Mutational analysis of domain II of flavonol 3-sulfotransferase
|
|
41971
|
Mutational analysis of domain II of flavonol 3-sulfotransferase
|
|
41972
|
Mutational analysis of domain II of flavonol 3-sulfotransferase
|
|
41973
|
Mutational analysis of domain II of flavonol 3-sulfotransferase
|
|
41974
|
Mutational analysis of domain II of flavonol 3-sulfotransferase
|
|
41975
|
Mutational analysis of domain II of flavonol 3-sulfotransferase
|
|
41976
|
Mutational analysis of domain II of flavonol 3-sulfotransferase
|
|
41977
|
Mutational analysis of domain II of flavonol 3-sulfotransferase
|
|
41978
|
Mutational analysis of domain II of flavonol 3-sulfotransferase
|
|
41979
|
Mutational analysis of domain II of flavonol 3-sulfotransferase
|
|
41980
|
Mutational analysis of domain II of flavonol 3-sulfotransferase
|
|
41981
|
Mutational analysis of domain II of flavonol 3-sulfotransferase
|
|
41982
|
Mutational analysis of domain II of flavonol 3-sulfotransferase
|
|
41983
|
Mutational analysis of domain II of flavonol 3-sulfotransferase
|
|
41984
|
Mutational analysis of domain II of flavonol 3-sulfotransferase
|
|
41985
|
Mutational analysis of domain II of flavonol 3-sulfotransferase
|
|
41986
|
Mutational analysis of domain II of flavonol 3-sulfotransferase
|
|
41987
|
Mutational analysis of domain II of flavonol 3-sulfotransferase
|
|
41988
|
Mutational analysis of domain II of flavonol 3-sulfotransferase
|
|
41989
|
Mutational analysis of domain II of flavonol 3-sulfotransferase
|
|
41990
|
Mutational analysis of domain II of flavonol 3-sulfotransferase
|
|
41991
|
Mutational analysis of domain II of flavonol 3-sulfotransferase
|
|
41992
|
Mutational analysis of domain II of flavonol 3-sulfotransferase
|
|
41993
|
Mutational analysis of domain II of flavonol 3-sulfotransferase
|
|
41994
|
Mutational analysis of domain II of flavonol 3-sulfotransferase
|
|
41995
|
Histidine decarboxylase of Lactobacillus 30a. VI. Mechanism of action and kinetic properties
|
|
41996
|
Histidine decarboxylase of Lactobacillus 30a. VI. Mechanism of action and kinetic properties
|
|
41997
|
Histidine decarboxylase of Lactobacillus 30a. VI. Mechanism of action and kinetic properties
|
|
41998
|
Identification of amino acid residues critical for catalysis and cosubstrate binding in the flavonol ...
|
|
41999
|
Identification of amino acid residues critical for catalysis and cosubstrate binding in the flavonol ...
|
|
42000
|
Identification of amino acid residues critical for catalysis and cosubstrate binding in the flavonol ...
|




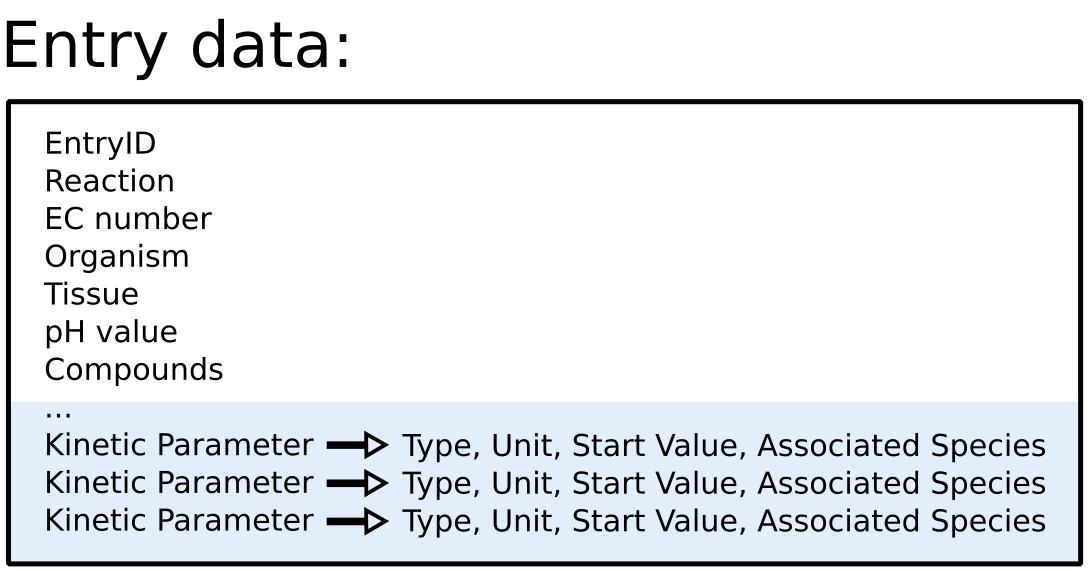
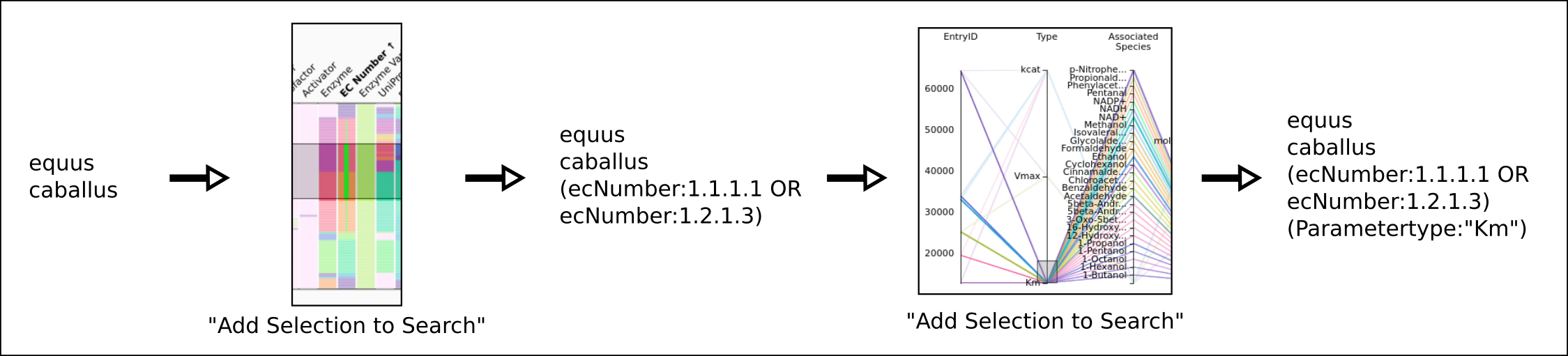 If the current search query is a refinement of the previous query "Use Previous Query (Go Back)" button can be used to perform the previous query again. Only one automatic step back is possible, but the query can be manually manipulated if desired.
If the current search query is a refinement of the previous query "Use Previous Query (Go Back)" button can be used to perform the previous query again. Only one automatic step back is possible, but the query can be manually manipulated if desired.