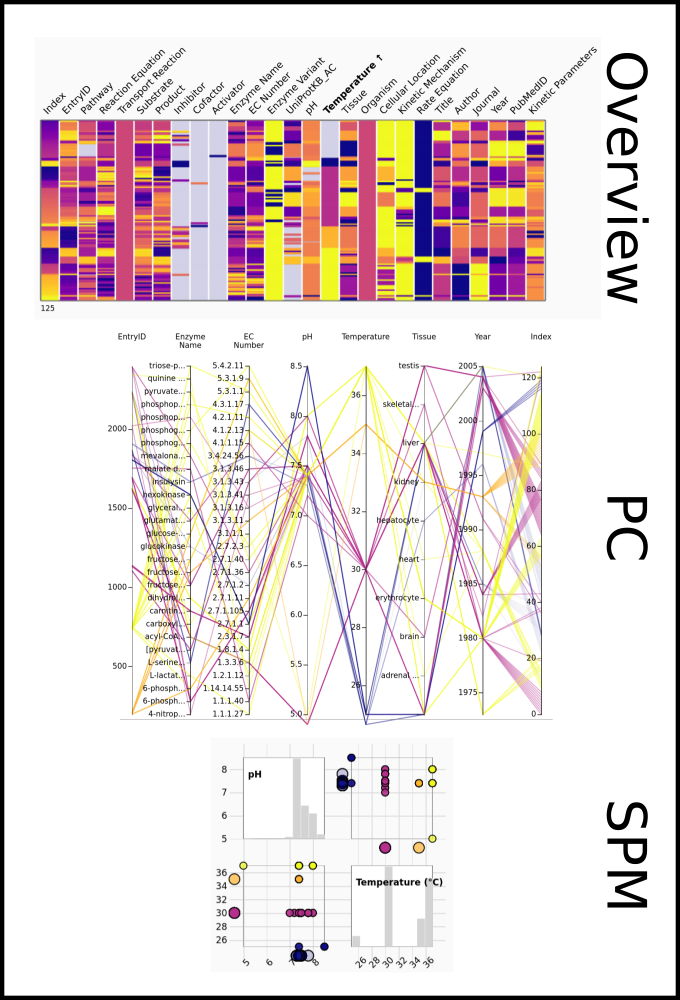
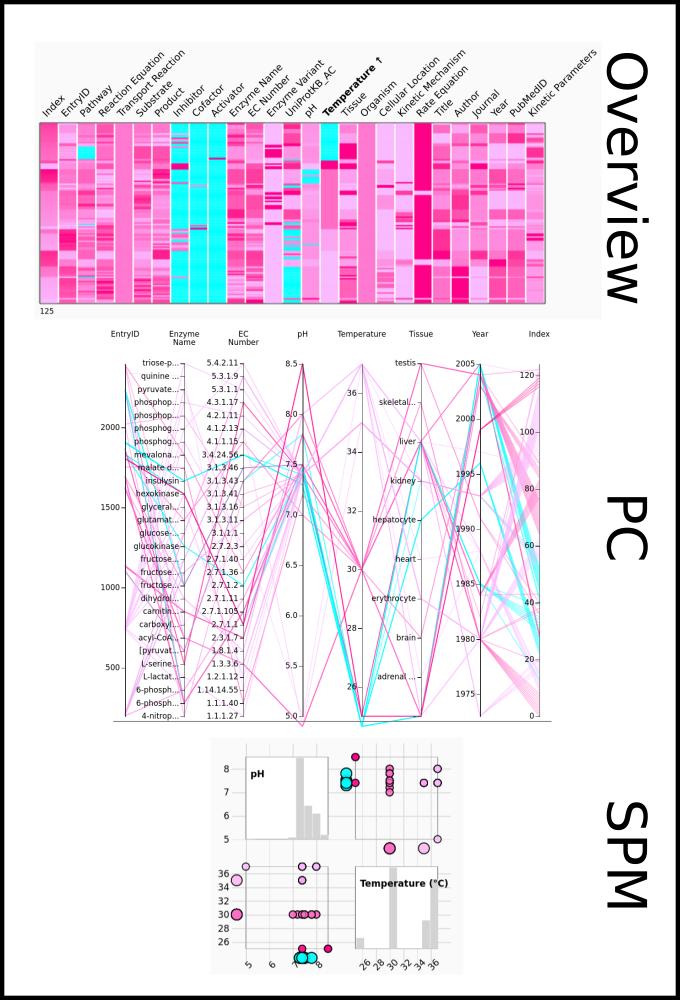
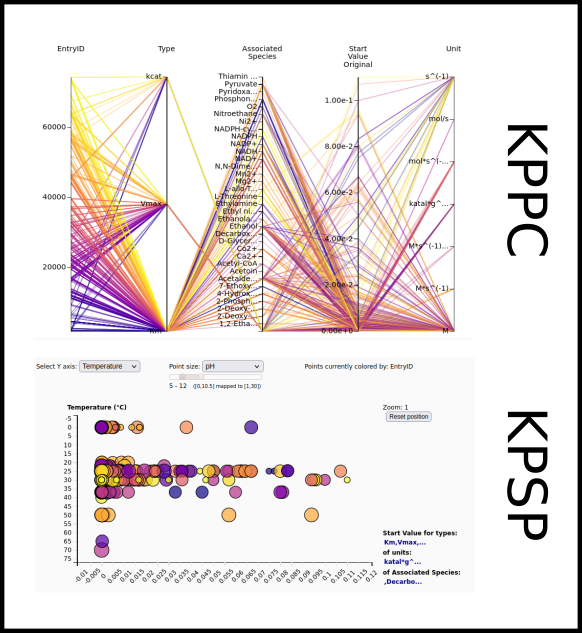
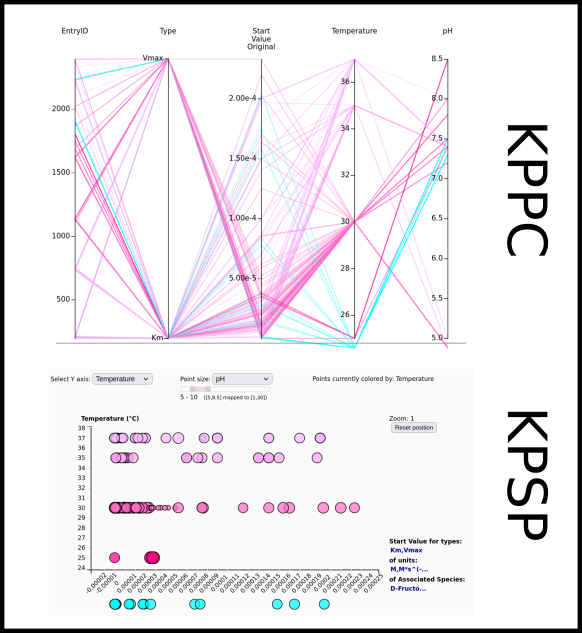
To improve SABIO-RK data interoperability, semantic markup was added to web pages as described and defined by
This structured information makes it easier to discover, collate and analyse our data.
|
22001
|
Crystal structure of rat biliverdin reductase
|
|
22002
|
Crystal structure of rat biliverdin reductase
|
|
22003
|
Crystal structure of rat biliverdin reductase
|
|
22004
|
Crystal structure of rat biliverdin reductase
|
|
22005
|
Crystal structure of rat biliverdin reductase
|
|
22006
|
Crystal structure of rat biliverdin reductase
|
|
22007
|
Crystal structure of rat biliverdin reductase
|
|
22008
|
Crystal structure of rat biliverdin reductase
|
|
22009
|
Crystal structure of rat biliverdin reductase
|
|
22010
|
Crystal structure of rat biliverdin reductase
|
|
22011
|
Crystal structure of rat biliverdin reductase
|
|
22012
|
Crystal structure of rat biliverdin reductase
|
|
22013
|
Crystal structure of rat biliverdin reductase
|
|
22014
|
Crystal structure of rat biliverdin reductase
|
|
22015
|
Kinetics and specificity of human B-cell glucokinase: relevance to hexose-induced insulin release.
|
|
22016
|
Kinetics and specificity of human B-cell glucokinase: relevance to hexose-induced insulin release.
|
|
22017
|
Kinetics and specificity of human B-cell glucokinase: relevance to hexose-induced insulin release.
|
|
22018
|
Kinetics and specificity of human B-cell glucokinase: relevance to hexose-induced insulin release.
|
|
22019
|
Kinetics and specificity of human B-cell glucokinase: relevance to hexose-induced insulin release.
|
|
22020
|
Kinetics and specificity of human B-cell glucokinase: relevance to hexose-induced insulin release.
|
|
22021
|
Class C beta-lactamases operate at the diffusion limit for turnover of their preferred cephalosporin ...
|
|
22022
|
Class C beta-lactamases operate at the diffusion limit for turnover of their preferred cephalosporin ...
|
|
22023
|
Class C beta-lactamases operate at the diffusion limit for turnover of their preferred cephalosporin ...
|
|
22024
|
Class C beta-lactamases operate at the diffusion limit for turnover of their preferred cephalosporin ...
|
|
22025
|
Class C beta-lactamases operate at the diffusion limit for turnover of their preferred cephalosporin ...
|
|
22026
|
Class C beta-lactamases operate at the diffusion limit for turnover of their preferred cephalosporin ...
|
|
22027
|
Class C beta-lactamases operate at the diffusion limit for turnover of their preferred cephalosporin ...
|
|
22028
|
Class C beta-lactamases operate at the diffusion limit for turnover of their preferred cephalosporin ...
|
|
22029
|
Class C beta-lactamases operate at the diffusion limit for turnover of their preferred cephalosporin ...
|
|
22030
|
Class C beta-lactamases operate at the diffusion limit for turnover of their preferred cephalosporin ...
|
|
22031
|
Class C beta-lactamases operate at the diffusion limit for turnover of their preferred cephalosporin ...
|
|
22032
|
Class C beta-lactamases operate at the diffusion limit for turnover of their preferred cephalosporin ...
|
|
22033
|
Class C beta-lactamases operate at the diffusion limit for turnover of their preferred cephalosporin ...
|
|
22034
|
Class C beta-lactamases operate at the diffusion limit for turnover of their preferred cephalosporin ...
|
|
22035
|
Class C beta-lactamases operate at the diffusion limit for turnover of their preferred cephalosporin ...
|
|
22036
|
Class C beta-lactamases operate at the diffusion limit for turnover of their preferred cephalosporin ...
|
|
22037
|
Class C beta-lactamases operate at the diffusion limit for turnover of their preferred cephalosporin ...
|
|
22038
|
Class C beta-lactamases operate at the diffusion limit for turnover of their preferred cephalosporin ...
|
|
22039
|
Class C beta-lactamases operate at the diffusion limit for turnover of their preferred cephalosporin ...
|
|
22040
|
Cysteinyl peptide inhibitors of Bacillus cereus zinc beta-lactamase
|
|
22041
|
Cysteinyl peptide inhibitors of Bacillus cereus zinc beta-lactamase
|
|
22042
|
Cysteinyl peptide inhibitors of Bacillus cereus zinc beta-lactamase
|
|
22043
|
Cysteinyl peptide inhibitors of Bacillus cereus zinc beta-lactamase
|
|
22044
|
Cysteinyl peptide inhibitors of Bacillus cereus zinc beta-lactamase
|
|
22045
|
Cysteinyl peptide inhibitors of Bacillus cereus zinc beta-lactamase
|
|
22046
|
Cysteinyl peptide inhibitors of Bacillus cereus zinc beta-lactamase
|
|
22047
|
Cysteinyl peptide inhibitors of Bacillus cereus zinc beta-lactamase
|
|
22048
|
Cysteinyl peptide inhibitors of Bacillus cereus zinc beta-lactamase
|
|
22049
|
Cysteinyl peptide inhibitors of Bacillus cereus zinc beta-lactamase
|
|
22050
|
Cysteinyl peptide inhibitors of Bacillus cereus zinc beta-lactamase
|
|
22051
|
Cysteinyl peptide inhibitors of Bacillus cereus zinc beta-lactamase
|
|
22052
|
Cysteinyl peptide inhibitors of Bacillus cereus zinc beta-lactamase
|
|
22053
|
Cysteinyl peptide inhibitors of Bacillus cereus zinc beta-lactamase
|
|
22054
|
Cysteinyl peptide inhibitors of Bacillus cereus zinc beta-lactamase
|
|
22055
|
Cysteinyl peptide inhibitors of Bacillus cereus zinc beta-lactamase
|
|
22056
|
Cysteinyl peptide inhibitors of Bacillus cereus zinc beta-lactamase
|
|
22057
|
Cysteinyl peptide inhibitors of Bacillus cereus zinc beta-lactamase
|
|
22058
|
Cysteinyl peptide inhibitors of Bacillus cereus zinc beta-lactamase
|
|
22059
|
Cysteinyl peptide inhibitors of Bacillus cereus zinc beta-lactamase
|
|
22060
|
Site-saturation mutagenesis of Tyr-105 reveals its importance in substrate stabilization and discrimination in ...
|
|
22061
|
Site-saturation mutagenesis of Tyr-105 reveals its importance in substrate stabilization and discrimination in ...
|
|
22062
|
Site-saturation mutagenesis of Tyr-105 reveals its importance in substrate stabilization and discrimination in ...
|
|
22063
|
Site-saturation mutagenesis of Tyr-105 reveals its importance in substrate stabilization and discrimination in ...
|
|
22064
|
Site-saturation mutagenesis of Tyr-105 reveals its importance in substrate stabilization and discrimination in ...
|
|
22065
|
Site-saturation mutagenesis of Tyr-105 reveals its importance in substrate stabilization and discrimination in ...
|
|
22066
|
Site-saturation mutagenesis of Tyr-105 reveals its importance in substrate stabilization and discrimination in ...
|
|
22067
|
Site-saturation mutagenesis of Tyr-105 reveals its importance in substrate stabilization and discrimination in ...
|
|
22068
|
Site-saturation mutagenesis of Tyr-105 reveals its importance in substrate stabilization and discrimination in ...
|
|
22069
|
Site-saturation mutagenesis of Tyr-105 reveals its importance in substrate stabilization and discrimination in ...
|
|
22070
|
Site-saturation mutagenesis of Tyr-105 reveals its importance in substrate stabilization and discrimination in ...
|
|
22071
|
Site-saturation mutagenesis of Tyr-105 reveals its importance in substrate stabilization and discrimination in ...
|
|
22072
|
Transport of alpha-aminoisobutyric acid by Streptococcus pyogenes and its derived L-form
|
|
22073
|
Transport of alpha-aminoisobutyric acid by Streptococcus pyogenes and its derived L-form
|
|
22074
|
Transport of alpha-aminoisobutyric acid by Streptococcus pyogenes and its derived L-form
|
|
22075
|
Transport of alpha-aminoisobutyric acid by Streptococcus pyogenes and its derived L-form
|
|
22076
|
Transport of alpha-aminoisobutyric acid by Streptococcus pyogenes and its derived L-form
|
|
22077
|
Transport of alpha-aminoisobutyric acid by Streptococcus pyogenes and its derived L-form
|
|
22078
|
Transport of alpha-aminoisobutyric acid by Streptococcus pyogenes and its derived L-form
|
|
22079
|
Transport of alpha-aminoisobutyric acid by Streptococcus pyogenes and its derived L-form
|
|
22080
|
Transport of alpha-aminoisobutyric acid by Streptococcus pyogenes and its derived L-form
|
|
22081
|
Transport of alpha-aminoisobutyric acid by Streptococcus pyogenes and its derived L-form
|
|
22082
|
Transport of alpha-aminoisobutyric acid by Streptococcus pyogenes and its derived L-form
|
|
22083
|
Transport of alpha-aminoisobutyric acid by Streptococcus pyogenes and its derived L-form
|
|
22084
|
Transport of alpha-aminoisobutyric acid by Streptococcus pyogenes and its derived L-form
|
|
22085
|
Transport of alpha-aminoisobutyric acid by Streptococcus pyogenes and its derived L-form
|
|
22086
|
The purification and properties of the alpha-glycerophosphate-oxidizing enzyme of Streptococcus faecalis 10C1
|
|
22087
|
Halibut muscle 3-phosphoglycerate kinase. Chemical and physical properties of the enzyme and its substrate ...
|
|
22088
|
Halibut muscle 3-phosphoglycerate kinase. Chemical and physical properties of the enzyme and its substrate ...
|
|
22089
|
Naturally occurring Phe151Leu substitution near a conserved folding module lowers stability of glutathione ...
|
|
22090
|
Naturally occurring Phe151Leu substitution near a conserved folding module lowers stability of glutathione ...
|
|
22091
|
Naturally occurring Phe151Leu substitution near a conserved folding module lowers stability of glutathione ...
|
|
22092
|
Naturally occurring Phe151Leu substitution near a conserved folding module lowers stability of glutathione ...
|
|
22093
|
Naturally occurring Phe151Leu substitution near a conserved folding module lowers stability of glutathione ...
|
|
22094
|
Naturally occurring Phe151Leu substitution near a conserved folding module lowers stability of glutathione ...
|
|
22095
|
Naturally occurring Phe151Leu substitution near a conserved folding module lowers stability of glutathione ...
|
|
22096
|
Naturally occurring Phe151Leu substitution near a conserved folding module lowers stability of glutathione ...
|
|
22097
|
Naturally occurring Phe151Leu substitution near a conserved folding module lowers stability of glutathione ...
|
|
22098
|
Naturally occurring Phe151Leu substitution near a conserved folding module lowers stability of glutathione ...
|
|
22099
|
Naturally occurring Phe151Leu substitution near a conserved folding module lowers stability of glutathione ...
|
|
22100
|
Naturally occurring Phe151Leu substitution near a conserved folding module lowers stability of glutathione ...
|
|
22101
|
Naturally occurring Phe151Leu substitution near a conserved folding module lowers stability of glutathione ...
|
|
22102
|
Naturally occurring Phe151Leu substitution near a conserved folding module lowers stability of glutathione ...
|
|
22103
|
Naturally occurring Phe151Leu substitution near a conserved folding module lowers stability of glutathione ...
|
|
22104
|
Naturally occurring Phe151Leu substitution near a conserved folding module lowers stability of glutathione ...
|
|
22105
|
Naturally occurring Phe151Leu substitution near a conserved folding module lowers stability of glutathione ...
|
|
22106
|
Naturally occurring Phe151Leu substitution near a conserved folding module lowers stability of glutathione ...
|
|
22107
|
Ceramide kinase, a novel lipid kinase. Molecular cloning and functional characterization
|
|
22108
|
Ceramide kinase, a novel lipid kinase. Molecular cloning and functional characterization
|
|
22109
|
Identification by mutagenesis of a conserved glutamate (Glu487) residue important for catalytic activity in ...
|
|
22110
|
Identification by mutagenesis of a conserved glutamate (Glu487) residue important for catalytic activity in ...
|
|
22111
|
Identification by mutagenesis of a conserved glutamate (Glu487) residue important for catalytic activity in ...
|
|
22112
|
Identification by mutagenesis of a conserved glutamate (Glu487) residue important for catalytic activity in ...
|
|
22113
|
Identification by mutagenesis of a conserved glutamate (Glu487) residue important for catalytic activity in ...
|
|
22114
|
Identification by mutagenesis of a conserved glutamate (Glu487) residue important for catalytic activity in ...
|
|
22115
|
Identification of conserved amino acid residues in rat liver carnitine palmitoyltransferase I critical for ...
|
|
22116
|
Identification of conserved amino acid residues in rat liver carnitine palmitoyltransferase I critical for ...
|
|
22117
|
Identification of conserved amino acid residues in rat liver carnitine palmitoyltransferase I critical for ...
|
|
22118
|
Identification of conserved amino acid residues in rat liver carnitine palmitoyltransferase I critical for ...
|
|
22119
|
Identification of conserved amino acid residues in rat liver carnitine palmitoyltransferase I critical for ...
|
|
22120
|
Identification of conserved amino acid residues in rat liver carnitine palmitoyltransferase I critical for ...
|
|
22121
|
Identification of conserved amino acid residues in rat liver carnitine palmitoyltransferase I critical for ...
|
|
22122
|
Identification of conserved amino acid residues in rat liver carnitine palmitoyltransferase I critical for ...
|
|
22123
|
Identification of conserved amino acid residues in rat liver carnitine palmitoyltransferase I critical for ...
|
|
22124
|
Identification of conserved amino acid residues in rat liver carnitine palmitoyltransferase I critical for ...
|
|
22125
|
Identification of conserved amino acid residues in rat liver carnitine palmitoyltransferase I critical for ...
|
|
22126
|
Identification of conserved amino acid residues in rat liver carnitine palmitoyltransferase I critical for ...
|
|
22127
|
Identification of conserved amino acid residues in rat liver carnitine palmitoyltransferase I critical for ...
|
|
22128
|
Identification of conserved amino acid residues in rat liver carnitine palmitoyltransferase I critical for ...
|
|
22129
|
Identification of conserved amino acid residues in rat liver carnitine palmitoyltransferase I critical for ...
|
|
22130
|
Identification of conserved amino acid residues in rat liver carnitine palmitoyltransferase I critical for ...
|
|
22131
|
Identification of conserved amino acid residues in rat liver carnitine palmitoyltransferase I critical for ...
|
|
22132
|
Identification of conserved amino acid residues in rat liver carnitine palmitoyltransferase I critical for ...
|
|
22133
|
Identification of conserved amino acid residues in rat liver carnitine palmitoyltransferase I critical for ...
|
|
22134
|
Identification of conserved amino acid residues in rat liver carnitine palmitoyltransferase I critical for ...
|
|
22135
|
Identification of conserved amino acid residues in rat liver carnitine palmitoyltransferase I critical for ...
|
|
22136
|
Identification of conserved amino acid residues in rat liver carnitine palmitoyltransferase I critical for ...
|
|
22137
|
Identification of conserved amino acid residues in rat liver carnitine palmitoyltransferase I critical for ...
|
|
22138
|
Identification of conserved amino acid residues in rat liver carnitine palmitoyltransferase I critical for ...
|
|
22139
|
Identification of conserved amino acid residues in rat liver carnitine palmitoyltransferase I critical for ...
|
|
22140
|
Identification of conserved amino acid residues in rat liver carnitine palmitoyltransferase I critical for ...
|
|
22141
|
Identification of conserved amino acid residues in rat liver carnitine palmitoyltransferase I critical for ...
|
|
22142
|
Cloning and characterization of mitochondrial 5-formyltetrahydrofolate cycloligase from higher plants
|
|
22143
|
Cloning and characterization of mitochondrial 5-formyltetrahydrofolate cycloligase from higher plants
|
|
22144
|
Cloning and characterization of mitochondrial 5-formyltetrahydrofolate cycloligase from higher plants
|
|
22145
|
Cloning and characterization of mitochondrial 5-formyltetrahydrofolate cycloligase from higher plants
|
|
22146
|
One step purification and characterization of the pyrrolidone carboxyl peptidase of Streptococcus pyogenes ...
|
|
22147
|
One step purification and characterization of the pyrrolidone carboxyl peptidase of Streptococcus pyogenes ...
|
|
22148
|
Adenine nucleotides affect the binding of 3-phosphoglycerate to pig muscle 3-phosphoglycerate kinase
|
|
22149
|
Adenine nucleotides affect the binding of 3-phosphoglycerate to pig muscle 3-phosphoglycerate kinase
|
|
22150
|
Adenine nucleotides affect the binding of 3-phosphoglycerate to pig muscle 3-phosphoglycerate kinase
|
|
22151
|
Adenine nucleotides affect the binding of 3-phosphoglycerate to pig muscle 3-phosphoglycerate kinase
|
|
22152
|
Adenine nucleotides affect the binding of 3-phosphoglycerate to pig muscle 3-phosphoglycerate kinase
|
|
22153
|
Probing the active site of aromatase with 2-methyl-substituted androstenedione analogs
|
|
22154
|
Probing the active site of aromatase with 2-methyl-substituted androstenedione analogs
|
|
22155
|
Probing the active site of aromatase with 2-methyl-substituted androstenedione analogs
|
|
22156
|
Probing the active site of aromatase with 2-methyl-substituted androstenedione analogs
|
|
22157
|
Probing the active site of aromatase with 2-methyl-substituted androstenedione analogs
|
|
22158
|
Probing the active site of aromatase with 2-methyl-substituted androstenedione analogs
|
|
22159
|
Probing the active site of aromatase with 2-methyl-substituted androstenedione analogs
|
|
22160
|
Probing the active site of aromatase with 2-methyl-substituted androstenedione analogs
|
|
22161
|
Probing the active site of aromatase with 2-methyl-substituted androstenedione analogs
|
|
22162
|
Probing the active site of aromatase with 2-methyl-substituted androstenedione analogs
|
|
22163
|
Probing the active site of aromatase with 2-methyl-substituted androstenedione analogs
|
|
22164
|
Probing the active site of aromatase with 2-methyl-substituted androstenedione analogs
|
|
22165
|
Activity of liver glutamine transaminase toward L-gamma-glutamyl hydrazones of alpha-keto acids
|
|
22166
|
Activity of liver glutamine transaminase toward L-gamma-glutamyl hydrazones of alpha-keto acids
|
|
22167
|
Evolution of enzymatic activities in the enolase superfamily: partitioning of reactive intermediates by ...
|
|
22168
|
Evolution of enzymatic activities in the enolase superfamily: partitioning of reactive intermediates by ...
|
|
22169
|
Evidence for cysteine persulfide as reaction product of L-Cyst(e)ine C-S-lyase (C-DES) from Synechocystis. ...
|
|
22170
|
Evidence for cysteine persulfide as reaction product of L-Cyst(e)ine C-S-lyase (C-DES) from Synechocystis. ...
|
|
22171
|
Evidence for cysteine persulfide as reaction product of L-Cyst(e)ine C-S-lyase (C-DES) from Synechocystis. ...
|
|
22172
|
Evidence for cysteine persulfide as reaction product of L-Cyst(e)ine C-S-lyase (C-DES) from Synechocystis. ...
|
|
22173
|
Evidence for cysteine persulfide as reaction product of L-Cyst(e)ine C-S-lyase (C-DES) from Synechocystis. ...
|
|
22174
|
Evidence for cysteine persulfide as reaction product of L-Cyst(e)ine C-S-lyase (C-DES) from Synechocystis. ...
|
|
22175
|
Evidence for cysteine persulfide as reaction product of L-Cyst(e)ine C-S-lyase (C-DES) from Synechocystis. ...
|
|
22176
|
Evidence for cysteine persulfide as reaction product of L-Cyst(e)ine C-S-lyase (C-DES) from Synechocystis. ...
|
|
22177
|
Evidence for cysteine persulfide as reaction product of L-Cyst(e)ine C-S-lyase (C-DES) from Synechocystis. ...
|
|
22178
|
Pantothenoylcysteine-4` -phosphate decarboxylase from horse liver
|
|
22179
|
Pantothenoylcysteine-4` -phosphate decarboxylase from horse liver
|
|
22180
|
Pantothenoylcysteine-4` -phosphate decarboxylase from horse liver
|
|
22181
|
Pantothenoylcysteine-4` -phosphate decarboxylase from horse liver
|
|
22182
|
Pantothenoylcysteine-4` -phosphate decarboxylase from horse liver
|
|
22183
|
Pantothenoylcysteine-4` -phosphate decarboxylase from horse liver
|
|
22184
|
Pantothenoylcysteine-4` -phosphate decarboxylase from horse liver
|
|
22185
|
Pantothenoylcysteine-4` -phosphate decarboxylase from horse liver
|
|
22186
|
Pantothenoylcysteine-4` -phosphate decarboxylase from horse liver
|
|
22187
|
Pantothenoylcysteine-4` -phosphate decarboxylase from horse liver
|
|
22188
|
Pantothenoylcysteine-4` -phosphate decarboxylase from horse liver
|
|
22189
|
Pantothenoylcysteine-4` -phosphate decarboxylase from horse liver
|
|
22190
|
Pantothenoylcysteine-4` -phosphate decarboxylase from horse liver
|
|
22191
|
Pantothenoylcysteine-4` -phosphate decarboxylase from horse liver
|
|
22192
|
Functional role of fatty acyl-coenzyme A synthetase in the transmembrane movement and activation of exogenous ...
|
|
22193
|
Functional role of fatty acyl-coenzyme A synthetase in the transmembrane movement and activation of exogenous ...
|
|
22194
|
Functional role of fatty acyl-coenzyme A synthetase in the transmembrane movement and activation of exogenous ...
|
|
22195
|
Functional role of fatty acyl-coenzyme A synthetase in the transmembrane movement and activation of exogenous ...
|
|
22196
|
Functional role of fatty acyl-coenzyme A synthetase in the transmembrane movement and activation of exogenous ...
|
|
22197
|
Functional role of fatty acyl-coenzyme A synthetase in the transmembrane movement and activation of exogenous ...
|
|
22198
|
Functional role of fatty acyl-coenzyme A synthetase in the transmembrane movement and activation of exogenous ...
|
|
22199
|
Functional role of fatty acyl-coenzyme A synthetase in the transmembrane movement and activation of exogenous ...
|
|
22200
|
Functional role of fatty acyl-coenzyme A synthetase in the transmembrane movement and activation of exogenous ...
|
|
22201
|
Functional role of fatty acyl-coenzyme A synthetase in the transmembrane movement and activation of exogenous ...
|
|
22202
|
Functional role of fatty acyl-coenzyme A synthetase in the transmembrane movement and activation of exogenous ...
|
|
22203
|
Functional role of fatty acyl-coenzyme A synthetase in the transmembrane movement and activation of exogenous ...
|
|
22204
|
Isolation, characterization, and kinetic properties of truncated forms of T4 DNA polymerase that exhibit 3'-5' ...
|
|
22205
|
Isolation, characterization, and kinetic properties of truncated forms of T4 DNA polymerase that exhibit 3'-5' ...
|
|
22206
|
Isolation, characterization, and kinetic properties of truncated forms of T4 DNA polymerase that exhibit 3'-5' ...
|
|
22207
|
Isolation, characterization, and kinetic properties of truncated forms of T4 DNA polymerase that exhibit 3'-5' ...
|
|
22208
|
Isolation, characterization, and kinetic properties of truncated forms of T4 DNA polymerase that exhibit 3'-5' ...
|
|
22209
|
Isolation, characterization, and kinetic properties of truncated forms of T4 DNA polymerase that exhibit 3'-5' ...
|
|
22210
|
Isolation, characterization, and kinetic properties of truncated forms of T4 DNA polymerase that exhibit 3'-5' ...
|
|
22211
|
Experimental evidence for the essential role of the C-terminal residue in the strict aminopeptidase activity ...
|
|
22212
|
Experimental evidence for the essential role of the C-terminal residue in the strict aminopeptidase activity ...
|
|
22213
|
Experimental evidence for the essential role of the C-terminal residue in the strict aminopeptidase activity ...
|
|
22214
|
Experimental evidence for the essential role of the C-terminal residue in the strict aminopeptidase activity ...
|
|
22215
|
Enolases from fluoride-sensitive and fluoride-resistant streptococci
|
|
22216
|
Enolases from fluoride-sensitive and fluoride-resistant streptococci
|
|
22217
|
Enolases from fluoride-sensitive and fluoride-resistant streptococci
|
|
22218
|
Enolases from fluoride-sensitive and fluoride-resistant streptococci
|
|
22219
|
Enolases from fluoride-sensitive and fluoride-resistant streptococci
|
|
22220
|
Enolases from fluoride-sensitive and fluoride-resistant streptococci
|
|
22221
|
Enolases from fluoride-sensitive and fluoride-resistant streptococci
|
|
22222
|
Enolases from fluoride-sensitive and fluoride-resistant streptococci
|
|
22223
|
Enolases from fluoride-sensitive and fluoride-resistant streptococci
|
|
22224
|
Enolases from fluoride-sensitive and fluoride-resistant streptococci
|
|
22225
|
Purification and partial characterisation of 4-aminobutyrate 2-ketoglutarate transaminase from human brain
|
|
22226
|
Purification and partial characterisation of 4-aminobutyrate 2-ketoglutarate transaminase from human brain
|
|
22227
|
Purification and partial characterisation of 4-aminobutyrate 2-ketoglutarate transaminase from human brain
|
|
22228
|
Purification and partial characterisation of 4-aminobutyrate 2-ketoglutarate transaminase from human brain
|
|
22229
|
Functional analysis of a group A streptococcal glycoside hydrolase Spy1600 from family 84 reveals it is a ...
|
|
22230
|
Functional analysis of a group A streptococcal glycoside hydrolase Spy1600 from family 84 reveals it is a ...
|
|
22231
|
Functional analysis of a group A streptococcal glycoside hydrolase Spy1600 from family 84 reveals it is a ...
|
|
22232
|
Functional analysis of a group A streptococcal glycoside hydrolase Spy1600 from family 84 reveals it is a ...
|
|
22233
|
Lysine-ketoglutarate reductase in human tissues
|
|
22234
|
Lysine-ketoglutarate reductase in human tissues
|
|
22235
|
Lysine-ketoglutarate reductase in human tissues
|
|
22236
|
Lysine-ketoglutarate reductase in human tissues
|
|
22237
|
Lysine-ketoglutarate reductase in human tissues
|
|
22238
|
Lysine-ketoglutarate reductase in human tissues
|
|
22239
|
Lysine-ketoglutarate reductase in human tissues
|
|
22240
|
Lysine-ketoglutarate reductase in human tissues
|
|
22241
|
Lysine-ketoglutarate reductase in human tissues
|
|
22242
|
Individual variation in hepatic aldehyde oxidase activity
|
|
22243
|
Individual variation in hepatic aldehyde oxidase activity
|
|
22244
|
Individual variation in hepatic aldehyde oxidase activity
|
|
22245
|
Individual variation in hepatic aldehyde oxidase activity
|
|
22246
|
Individual variation in hepatic aldehyde oxidase activity
|
|
22247
|
Individual variation in hepatic aldehyde oxidase activity
|
|
22248
|
Individual variation in hepatic aldehyde oxidase activity
|
|
22249
|
Individual variation in hepatic aldehyde oxidase activity
|
|
22250
|
Individual variation in hepatic aldehyde oxidase activity
|
|
22251
|
Individual variation in hepatic aldehyde oxidase activity
|
|
22252
|
Individual variation in hepatic aldehyde oxidase activity
|
|
22253
|
Individual variation in hepatic aldehyde oxidase activity
|
|
22254
|
Individual variation in hepatic aldehyde oxidase activity
|
|
22255
|
Individual variation in hepatic aldehyde oxidase activity
|
|
22256
|
Individual variation in hepatic aldehyde oxidase activity
|
|
22257
|
Individual variation in hepatic aldehyde oxidase activity
|
|
22258
|
Individual variation in hepatic aldehyde oxidase activity
|
|
22259
|
Individual variation in hepatic aldehyde oxidase activity
|
|
22260
|
Individual variation in hepatic aldehyde oxidase activity
|
|
22261
|
Individual variation in hepatic aldehyde oxidase activity
|
|
22262
|
Individual variation in hepatic aldehyde oxidase activity
|
|
22263
|
Individual variation in hepatic aldehyde oxidase activity
|
|
22264
|
Individual variation in hepatic aldehyde oxidase activity
|
|
22265
|
Individual variation in hepatic aldehyde oxidase activity
|
|
22266
|
Individual variation in hepatic aldehyde oxidase activity
|
|
22267
|
Individual variation in hepatic aldehyde oxidase activity
|
|
22268
|
Individual variation in hepatic aldehyde oxidase activity
|
|
22269
|
Individual variation in hepatic aldehyde oxidase activity
|
|
22270
|
Individual variation in hepatic aldehyde oxidase activity
|
|
22271
|
Individual variation in hepatic aldehyde oxidase activity
|
|
22272
|
Individual variation in hepatic aldehyde oxidase activity
|
|
22273
|
Individual variation in hepatic aldehyde oxidase activity
|
|
22274
|
Individual variation in hepatic aldehyde oxidase activity
|
|
22275
|
Individual variation in hepatic aldehyde oxidase activity
|
|
22276
|
Individual variation in hepatic aldehyde oxidase activity
|
|
22277
|
Individual variation in hepatic aldehyde oxidase activity
|
|
22278
|
Individual variation in hepatic aldehyde oxidase activity
|
|
22279
|
Individual variation in hepatic aldehyde oxidase activity
|
|
22280
|
Individual variation in hepatic aldehyde oxidase activity
|
|
22281
|
Properties and kinetic analysis of UDP-glucose dehydrogenase from group A streptococci. Irreversible ...
|
|
22282
|
Properties and kinetic analysis of UDP-glucose dehydrogenase from group A streptococci. Irreversible ...
|
|
22283
|
Properties and kinetic analysis of UDP-glucose dehydrogenase from group A streptococci. Irreversible ...
|
|
22284
|
Properties and kinetic analysis of UDP-glucose dehydrogenase from group A streptococci. Irreversible ...
|
|
22285
|
Properties and kinetic analysis of UDP-glucose dehydrogenase from group A streptococci. Irreversible ...
|
|
22286
|
Human IMP dehydrogenase. Kinetics and regulatory properties
|
|
22287
|
Human IMP dehydrogenase. Kinetics and regulatory properties
|
|
22288
|
Human IMP dehydrogenase. Kinetics and regulatory properties
|
|
22289
|
Human IMP dehydrogenase. Kinetics and regulatory properties
|
|
22290
|
Human IMP dehydrogenase. Kinetics and regulatory properties
|
|
22291
|
Human IMP dehydrogenase. Kinetics and regulatory properties
|
|
22292
|
Human IMP dehydrogenase. Kinetics and regulatory properties
|
|
22293
|
Pig liver carnitine palmitoyltransferase. Chimera studies show that both the N- and C-terminal regions of the ...
|
|
22294
|
Pig liver carnitine palmitoyltransferase. Chimera studies show that both the N- and C-terminal regions of the ...
|
|
22295
|
Pig liver carnitine palmitoyltransferase. Chimera studies show that both the N- and C-terminal regions of the ...
|
|
22296
|
Pig liver carnitine palmitoyltransferase. Chimera studies show that both the N- and C-terminal regions of the ...
|
|
22297
|
Pig liver carnitine palmitoyltransferase. Chimera studies show that both the N- and C-terminal regions of the ...
|
|
22298
|
Pig liver carnitine palmitoyltransferase. Chimera studies show that both the N- and C-terminal regions of the ...
|
|
22299
|
Pig liver carnitine palmitoyltransferase. Chimera studies show that both the N- and C-terminal regions of the ...
|
|
22300
|
Pig liver carnitine palmitoyltransferase. Chimera studies show that both the N- and C-terminal regions of the ...
|
|
22301
|
Pig liver carnitine palmitoyltransferase. Chimera studies show that both the N- and C-terminal regions of the ...
|
|
22302
|
Pig liver carnitine palmitoyltransferase. Chimera studies show that both the N- and C-terminal regions of the ...
|
|
22303
|
Pig liver carnitine palmitoyltransferase. Chimera studies show that both the N- and C-terminal regions of the ...
|
|
22304
|
Molecular diversity of a putative virulence factor: purification and characterization of isoforms of an ...
|
|
22305
|
Identification of positive and negative determinants of malonyl-CoA sensitivity and carnitine affinity within ...
|
|
22306
|
Identification of positive and negative determinants of malonyl-CoA sensitivity and carnitine affinity within ...
|
|
22307
|
Identification of positive and negative determinants of malonyl-CoA sensitivity and carnitine affinity within ...
|
|
22308
|
Identification of positive and negative determinants of malonyl-CoA sensitivity and carnitine affinity within ...
|
|
22309
|
Identification of positive and negative determinants of malonyl-CoA sensitivity and carnitine affinity within ...
|
|
22310
|
Identification of positive and negative determinants of malonyl-CoA sensitivity and carnitine affinity within ...
|
|
22311
|
Identification of positive and negative determinants of malonyl-CoA sensitivity and carnitine affinity within ...
|
|
22312
|
Identification of positive and negative determinants of malonyl-CoA sensitivity and carnitine affinity within ...
|
|
22313
|
Identification of positive and negative determinants of malonyl-CoA sensitivity and carnitine affinity within ...
|
|
22314
|
Identification of positive and negative determinants of malonyl-CoA sensitivity and carnitine affinity within ...
|
|
22315
|
Identification of positive and negative determinants of malonyl-CoA sensitivity and carnitine affinity within ...
|
|
22316
|
Identification of positive and negative determinants of malonyl-CoA sensitivity and carnitine affinity within ...
|
|
22317
|
Identification of positive and negative determinants of malonyl-CoA sensitivity and carnitine affinity within ...
|
|
22318
|
Identification of positive and negative determinants of malonyl-CoA sensitivity and carnitine affinity within ...
|
|
22319
|
Identification of positive and negative determinants of malonyl-CoA sensitivity and carnitine affinity within ...
|
|
22320
|
Identification of positive and negative determinants of malonyl-CoA sensitivity and carnitine affinity within ...
|
|
22321
|
Identification of positive and negative determinants of malonyl-CoA sensitivity and carnitine affinity within ...
|
|
22322
|
Identification of positive and negative determinants of malonyl-CoA sensitivity and carnitine affinity within ...
|
|
22323
|
Identification of positive and negative determinants of malonyl-CoA sensitivity and carnitine affinity within ...
|
|
22324
|
Identification of positive and negative determinants of malonyl-CoA sensitivity and carnitine affinity within ...
|
|
22325
|
Identification of positive and negative determinants of malonyl-CoA sensitivity and carnitine affinity within ...
|
|
22326
|
Identification of positive and negative determinants of malonyl-CoA sensitivity and carnitine affinity within ...
|
|
22327
|
Identification of positive and negative determinants of malonyl-CoA sensitivity and carnitine affinity within ...
|
|
22328
|
Identification of positive and negative determinants of malonyl-CoA sensitivity and carnitine affinity within ...
|
|
22329
|
Identification of positive and negative determinants of malonyl-CoA sensitivity and carnitine affinity within ...
|
|
22330
|
Identification of positive and negative determinants of malonyl-CoA sensitivity and carnitine affinity within ...
|
|
22331
|
Identification of positive and negative determinants of malonyl-CoA sensitivity and carnitine affinity within ...
|
|
22332
|
Sulfonamide resistance in Streptococcus pyogenes is associated with differences in the amino acid sequence of ...
|
|
22333
|
Sulfonamide resistance in Streptococcus pyogenes is associated with differences in the amino acid sequence of ...
|
|
22334
|
Sulfonamide resistance in Streptococcus pyogenes is associated with differences in the amino acid sequence of ...
|
|
22335
|
Sulfonamide resistance in Streptococcus pyogenes is associated with differences in the amino acid sequence of ...
|
|
22336
|
Metabolic activation of pradefovir by CYP3A4 and its potential as an inhibitor or inducer
|
|
22337
|
Metabolic activation of pradefovir by CYP3A4 and its potential as an inhibitor or inducer
|
|
22338
|
Glucose phosphorylation. Site-directed mutations which impair the catalytic function of hexokinase
|
|
22339
|
Glucose phosphorylation. Site-directed mutations which impair the catalytic function of hexokinase
|
|
22340
|
Glucose phosphorylation. Site-directed mutations which impair the catalytic function of hexokinase
|
|
22341
|
Glucose phosphorylation. Site-directed mutations which impair the catalytic function of hexokinase
|
|
22342
|
Glucose phosphorylation. Site-directed mutations which impair the catalytic function of hexokinase
|
|
22343
|
Glucose phosphorylation. Site-directed mutations which impair the catalytic function of hexokinase
|
|
22344
|
Glucose phosphorylation. Site-directed mutations which impair the catalytic function of hexokinase
|
|
22345
|
Glucose phosphorylation. Site-directed mutations which impair the catalytic function of hexokinase
|
|
22346
|
Glucose phosphorylation. Site-directed mutations which impair the catalytic function of hexokinase
|
|
22347
|
Glucose phosphorylation. Site-directed mutations which impair the catalytic function of hexokinase
|
|
22348
|
Glucose phosphorylation. Site-directed mutations which impair the catalytic function of hexokinase
|
|
22349
|
Glucose phosphorylation. Site-directed mutations which impair the catalytic function of hexokinase
|
|
22350
|
The vertebrate alcohol dehydrogenase system: variable class II type form elucidates separate stages of ...
|
|
22351
|
The vertebrate alcohol dehydrogenase system: variable class II type form elucidates separate stages of ...
|
|
22352
|
The vertebrate alcohol dehydrogenase system: variable class II type form elucidates separate stages of ...
|
|
22353
|
The vertebrate alcohol dehydrogenase system: variable class II type form elucidates separate stages of ...
|
|
22354
|
The vertebrate alcohol dehydrogenase system: variable class II type form elucidates separate stages of ...
|
|
22355
|
Characterization of chicken-liver glutathione S-transferase (GST) A1-1 and A2-2 isoenzymes and their ...
|
|
22356
|
Characterization of chicken-liver glutathione S-transferase (GST) A1-1 and A2-2 isoenzymes and their ...
|
|
22357
|
Characterization of chicken-liver glutathione S-transferase (GST) A1-1 and A2-2 isoenzymes and their ...
|
|
22358
|
Characterization of chicken-liver glutathione S-transferase (GST) A1-1 and A2-2 isoenzymes and their ...
|
|
22359
|
Characterization of chicken-liver glutathione S-transferase (GST) A1-1 and A2-2 isoenzymes and their ...
|
|
22360
|
Characterization of chicken-liver glutathione S-transferase (GST) A1-1 and A2-2 isoenzymes and their ...
|
|
22361
|
Characterization of chicken-liver glutathione S-transferase (GST) A1-1 and A2-2 isoenzymes and their ...
|
|
22362
|
Characterization of chicken-liver glutathione S-transferase (GST) A1-1 and A2-2 isoenzymes and their ...
|
|
22363
|
Characterization of chicken-liver glutathione S-transferase (GST) A1-1 and A2-2 isoenzymes and their ...
|
|
22364
|
Characterization of chicken-liver glutathione S-transferase (GST) A1-1 and A2-2 isoenzymes and their ...
|
|
22365
|
Characterization of chicken-liver glutathione S-transferase (GST) A1-1 and A2-2 isoenzymes and their ...
|
|
22366
|
Characterization of chicken-liver glutathione S-transferase (GST) A1-1 and A2-2 isoenzymes and their ...
|
|
22367
|
Characterization of chicken-liver glutathione S-transferase (GST) A1-1 and A2-2 isoenzymes and their ...
|
|
22368
|
Characterization of chicken-liver glutathione S-transferase (GST) A1-1 and A2-2 isoenzymes and their ...
|
|
22369
|
A single-residue exchange gives human recombinant beta beta alcohol dehydrogenase gamma gamma isozyme ...
|
|
22370
|
A single-residue exchange gives human recombinant beta beta alcohol dehydrogenase gamma gamma isozyme ...
|
|
22371
|
A single-residue exchange gives human recombinant beta beta alcohol dehydrogenase gamma gamma isozyme ...
|
|
22372
|
A single-residue exchange gives human recombinant beta beta alcohol dehydrogenase gamma gamma isozyme ...
|
|
22373
|
A single-residue exchange gives human recombinant beta beta alcohol dehydrogenase gamma gamma isozyme ...
|
|
22374
|
A single-residue exchange gives human recombinant beta beta alcohol dehydrogenase gamma gamma isozyme ...
|
|
22375
|
A single-residue exchange gives human recombinant beta beta alcohol dehydrogenase gamma gamma isozyme ...
|
|
22376
|
A single-residue exchange gives human recombinant beta beta alcohol dehydrogenase gamma gamma isozyme ...
|
|
22377
|
A single-residue exchange gives human recombinant beta beta alcohol dehydrogenase gamma gamma isozyme ...
|
|
22378
|
A single-residue exchange gives human recombinant beta beta alcohol dehydrogenase gamma gamma isozyme ...
|
|
22379
|
A single-residue exchange gives human recombinant beta beta alcohol dehydrogenase gamma gamma isozyme ...
|
|
22380
|
A single-residue exchange gives human recombinant beta beta alcohol dehydrogenase gamma gamma isozyme ...
|
|
22381
|
A single-residue exchange gives human recombinant beta beta alcohol dehydrogenase gamma gamma isozyme ...
|
|
22382
|
A single-residue exchange gives human recombinant beta beta alcohol dehydrogenase gamma gamma isozyme ...
|
|
22383
|
A single-residue exchange gives human recombinant beta beta alcohol dehydrogenase gamma gamma isozyme ...
|
|
22384
|
A single-residue exchange gives human recombinant beta beta alcohol dehydrogenase gamma gamma isozyme ...
|
|
22385
|
A single-residue exchange gives human recombinant beta beta alcohol dehydrogenase gamma gamma isozyme ...
|
|
22386
|
Cloning and expression of the liver and muscle isoforms of ovine carnitine palmitoyltransferase 1: residues ...
|
|
22387
|
Cloning and expression of the liver and muscle isoforms of ovine carnitine palmitoyltransferase 1: residues ...
|
|
22388
|
Cloning and expression of the liver and muscle isoforms of ovine carnitine palmitoyltransferase 1: residues ...
|
|
22389
|
Cloning and expression of the liver and muscle isoforms of ovine carnitine palmitoyltransferase 1: residues ...
|
|
22390
|
Cloning and expression of the liver and muscle isoforms of ovine carnitine palmitoyltransferase 1: residues ...
|
|
22391
|
Cloning and expression of the liver and muscle isoforms of ovine carnitine palmitoyltransferase 1: residues ...
|
|
22392
|
Cloning and expression of the liver and muscle isoforms of ovine carnitine palmitoyltransferase 1: residues ...
|
|
22393
|
Cloning and expression of the liver and muscle isoforms of ovine carnitine palmitoyltransferase 1: residues ...
|
|
22394
|
Cloning and expression of the liver and muscle isoforms of ovine carnitine palmitoyltransferase 1: residues ...
|
|
22395
|
Cloning and expression of the liver and muscle isoforms of ovine carnitine palmitoyltransferase 1: residues ...
|
|
22396
|
The mature size of rat 4-aminobutyrate aminotransferase is different in liver and brain
|
|
22397
|
The mature size of rat 4-aminobutyrate aminotransferase is different in liver and brain
|
|
22398
|
DPNH peroxidase: effector activities of DPN
|
|
22399
|
Characterization and identification of an epidermal-growth-factor-activated phospholipase A2
|
|
22400
|
Effects of alternate RNA splicing on glucokinase isoform activities in the pancreatic islet, liver, and ...
|
|
22401
|
Effects of alternate RNA splicing on glucokinase isoform activities in the pancreatic islet, liver, and ...
|
|
22402
|
Effects of alternate RNA splicing on glucokinase isoform activities in the pancreatic islet, liver, and ...
|
|
22403
|
Effects of alternate RNA splicing on glucokinase isoform activities in the pancreatic islet, liver, and ...
|
|
22404
|
Effects of alternate RNA splicing on glucokinase isoform activities in the pancreatic islet, liver, and ...
|
|
22405
|
A bifunctional enzyme catalyzes the first two steps in N-acetylneuraminic acid biosynthesis of rat liver. ...
|
|
22406
|
A bifunctional enzyme catalyzes the first two steps in N-acetylneuraminic acid biosynthesis of rat liver. ...
|
|
22407
|
A bifunctional enzyme catalyzes the first two steps in N-acetylneuraminic acid biosynthesis of rat liver. ...
|
|
22408
|
A bifunctional enzyme catalyzes the first two steps in N-acetylneuraminic acid biosynthesis of rat liver. ...
|
|
22409
|
A bifunctional enzyme catalyzes the first two steps in N-acetylneuraminic acid biosynthesis of rat liver. ...
|
|
22410
|
A bifunctional enzyme catalyzes the first two steps in N-acetylneuraminic acid biosynthesis of rat liver. ...
|
|
22411
|
Some observations on the NADP+-linked oxidation of methylglyoxal catalysed by 2-Oxoaldehyde dehydrogenase
|
|
22412
|
Some observations on the NADP+-linked oxidation of methylglyoxal catalysed by 2-Oxoaldehyde dehydrogenase
|
|
22413
|
Some observations on the NADP+-linked oxidation of methylglyoxal catalysed by 2-Oxoaldehyde dehydrogenase
|
|
22414
|
Some observations on the NADP+-linked oxidation of methylglyoxal catalysed by 2-Oxoaldehyde dehydrogenase
|
|
22415
|
Some observations on the NADP+-linked oxidation of methylglyoxal catalysed by 2-Oxoaldehyde dehydrogenase
|
|
22416
|
Some observations on the NADP+-linked oxidation of methylglyoxal catalysed by 2-Oxoaldehyde dehydrogenase
|
|
22417
|
Inhibitors of DNA polymerase III as novel antimicrobial agents against gram-positive eubacteria
|
|
22418
|
Inhibitors of DNA polymerase III as novel antimicrobial agents against gram-positive eubacteria
|
|
22419
|
Inhibitors of DNA polymerase III as novel antimicrobial agents against gram-positive eubacteria
|
|
22420
|
Inhibitors of DNA polymerase III as novel antimicrobial agents against gram-positive eubacteria
|
|
22421
|
Inhibitors of DNA polymerase III as novel antimicrobial agents against gram-positive eubacteria
|
|
22422
|
Inhibitors of DNA polymerase III as novel antimicrobial agents against gram-positive eubacteria
|
|
22423
|
Inhibitors of DNA polymerase III as novel antimicrobial agents against gram-positive eubacteria
|
|
22424
|
Inhibitors of DNA polymerase III as novel antimicrobial agents against gram-positive eubacteria
|
|
22425
|
Inhibitors of DNA polymerase III as novel antimicrobial agents against gram-positive eubacteria
|
|
22426
|
Inhibitors of DNA polymerase III as novel antimicrobial agents against gram-positive eubacteria
|
|
22427
|
Inhibitors of DNA polymerase III as novel antimicrobial agents against gram-positive eubacteria
|
|
22428
|
Inhibitors of DNA polymerase III as novel antimicrobial agents against gram-positive eubacteria
|
|
22429
|
Inhibitors of DNA polymerase III as novel antimicrobial agents against gram-positive eubacteria
|
|
22430
|
Inhibitors of DNA polymerase III as novel antimicrobial agents against gram-positive eubacteria
|
|
22431
|
Inhibitors of DNA polymerase III as novel antimicrobial agents against gram-positive eubacteria
|
|
22432
|
Inhibitors of DNA polymerase III as novel antimicrobial agents against gram-positive eubacteria
|
|
22433
|
Inhibitors of DNA polymerase III as novel antimicrobial agents against gram-positive eubacteria
|
|
22434
|
Inhibitors of DNA polymerase III as novel antimicrobial agents against gram-positive eubacteria
|
|
22435
|
Inhibitors of DNA polymerase III as novel antimicrobial agents against gram-positive eubacteria
|
|
22436
|
Inhibitors of DNA polymerase III as novel antimicrobial agents against gram-positive eubacteria
|
|
22437
|
Inhibitors of DNA polymerase III as novel antimicrobial agents against gram-positive eubacteria
|
|
22438
|
Inhibitors of DNA polymerase III as novel antimicrobial agents against gram-positive eubacteria
|
|
22439
|
Inhibitors of DNA polymerase III as novel antimicrobial agents against gram-positive eubacteria
|
|
22440
|
Inhibitors of DNA polymerase III as novel antimicrobial agents against gram-positive eubacteria
|
|
22441
|
Inhibitors of DNA polymerase III as novel antimicrobial agents against gram-positive eubacteria
|
|
22442
|
Inhibitors of DNA polymerase III as novel antimicrobial agents against gram-positive eubacteria
|
|
22443
|
Inhibitors of DNA polymerase III as novel antimicrobial agents against gram-positive eubacteria
|
|
22444
|
Inhibitors of DNA polymerase III as novel antimicrobial agents against gram-positive eubacteria
|
|
22445
|
Inhibitors of DNA polymerase III as novel antimicrobial agents against gram-positive eubacteria
|
|
22446
|
Inhibitors of DNA polymerase III as novel antimicrobial agents against gram-positive eubacteria
|
|
22447
|
Inhibitors of DNA polymerase III as novel antimicrobial agents against gram-positive eubacteria
|
|
22448
|
Inhibitors of DNA polymerase III as novel antimicrobial agents against gram-positive eubacteria
|
|
22449
|
Inhibitors of DNA polymerase III as novel antimicrobial agents against gram-positive eubacteria
|
|
22450
|
Inhibitors of DNA polymerase III as novel antimicrobial agents against gram-positive eubacteria
|
|
22451
|
Inhibitors of DNA polymerase III as novel antimicrobial agents against gram-positive eubacteria
|
|
22452
|
Inhibitors of DNA polymerase III as novel antimicrobial agents against gram-positive eubacteria
|
|
22453
|
Inhibitors of DNA polymerase III as novel antimicrobial agents against gram-positive eubacteria
|
|
22454
|
Inhibitors of DNA polymerase III as novel antimicrobial agents against gram-positive eubacteria
|
|
22455
|
Inhibitors of DNA polymerase III as novel antimicrobial agents against gram-positive eubacteria
|
|
22456
|
Inhibitors of DNA polymerase III as novel antimicrobial agents against gram-positive eubacteria
|
|
22457
|
Inhibitors of DNA polymerase III as novel antimicrobial agents against gram-positive eubacteria
|
|
22458
|
Inhibitors of DNA polymerase III as novel antimicrobial agents against gram-positive eubacteria
|
|
22459
|
Inhibitors of DNA polymerase III as novel antimicrobial agents against gram-positive eubacteria
|
|
22460
|
Inhibitors of DNA polymerase III as novel antimicrobial agents against gram-positive eubacteria
|
|
22461
|
Inhibitors of DNA polymerase III as novel antimicrobial agents against gram-positive eubacteria
|
|
22462
|
Lid L11 of the glutamine amidotransferase domain of CTP synthase mediates allosteric GTP activation of ...
|
|
22463
|
Lid L11 of the glutamine amidotransferase domain of CTP synthase mediates allosteric GTP activation of ...
|
|
22464
|
Lid L11 of the glutamine amidotransferase domain of CTP synthase mediates allosteric GTP activation of ...
|
|
22465
|
Lid L11 of the glutamine amidotransferase domain of CTP synthase mediates allosteric GTP activation of ...
|
|
22466
|
Lid L11 of the glutamine amidotransferase domain of CTP synthase mediates allosteric GTP activation of ...
|
|
22467
|
Lid L11 of the glutamine amidotransferase domain of CTP synthase mediates allosteric GTP activation of ...
|
|
22468
|
Lid L11 of the glutamine amidotransferase domain of CTP synthase mediates allosteric GTP activation of ...
|
|
22469
|
Lid L11 of the glutamine amidotransferase domain of CTP synthase mediates allosteric GTP activation of ...
|
|
22470
|
Lid L11 of the glutamine amidotransferase domain of CTP synthase mediates allosteric GTP activation of ...
|
|
22471
|
Lid L11 of the glutamine amidotransferase domain of CTP synthase mediates allosteric GTP activation of ...
|
|
22472
|
Lid L11 of the glutamine amidotransferase domain of CTP synthase mediates allosteric GTP activation of ...
|
|
22473
|
Lid L11 of the glutamine amidotransferase domain of CTP synthase mediates allosteric GTP activation of ...
|
|
22474
|
Lid L11 of the glutamine amidotransferase domain of CTP synthase mediates allosteric GTP activation of ...
|
|
22475
|
Lid L11 of the glutamine amidotransferase domain of CTP synthase mediates allosteric GTP activation of ...
|
|
22476
|
Lid L11 of the glutamine amidotransferase domain of CTP synthase mediates allosteric GTP activation of ...
|
|
22477
|
Lid L11 of the glutamine amidotransferase domain of CTP synthase mediates allosteric GTP activation of ...
|
|
22478
|
Lid L11 of the glutamine amidotransferase domain of CTP synthase mediates allosteric GTP activation of ...
|
|
22479
|
Lid L11 of the glutamine amidotransferase domain of CTP synthase mediates allosteric GTP activation of ...
|
|
22480
|
Lid L11 of the glutamine amidotransferase domain of CTP synthase mediates allosteric GTP activation of ...
|
|
22481
|
Lid L11 of the glutamine amidotransferase domain of CTP synthase mediates allosteric GTP activation of ...
|
|
22482
|
Lid L11 of the glutamine amidotransferase domain of CTP synthase mediates allosteric GTP activation of ...
|
|
22483
|
Lid L11 of the glutamine amidotransferase domain of CTP synthase mediates allosteric GTP activation of ...
|
|
22484
|
Lid L11 of the glutamine amidotransferase domain of CTP synthase mediates allosteric GTP activation of ...
|
|
22485
|
Lid L11 of the glutamine amidotransferase domain of CTP synthase mediates allosteric GTP activation of ...
|
|
22486
|
Lid L11 of the glutamine amidotransferase domain of CTP synthase mediates allosteric GTP activation of ...
|
|
22487
|
Lid L11 of the glutamine amidotransferase domain of CTP synthase mediates allosteric GTP activation of ...
|
|
22488
|
Lid L11 of the glutamine amidotransferase domain of CTP synthase mediates allosteric GTP activation of ...
|
|
22489
|
Lid L11 of the glutamine amidotransferase domain of CTP synthase mediates allosteric GTP activation of ...
|
|
22490
|
Lid L11 of the glutamine amidotransferase domain of CTP synthase mediates allosteric GTP activation of ...
|
|
22491
|
Lid L11 of the glutamine amidotransferase domain of CTP synthase mediates allosteric GTP activation of ...
|
|
22492
|
Lid L11 of the glutamine amidotransferase domain of CTP synthase mediates allosteric GTP activation of ...
|
|
22493
|
Lid L11 of the glutamine amidotransferase domain of CTP synthase mediates allosteric GTP activation of ...
|
|
22494
|
Lid L11 of the glutamine amidotransferase domain of CTP synthase mediates allosteric GTP activation of ...
|
|
22495
|
Lid L11 of the glutamine amidotransferase domain of CTP synthase mediates allosteric GTP activation of ...
|
|
22496
|
Lid L11 of the glutamine amidotransferase domain of CTP synthase mediates allosteric GTP activation of ...
|
|
22497
|
Lid L11 of the glutamine amidotransferase domain of CTP synthase mediates allosteric GTP activation of ...
|
|
22498
|
Lid L11 of the glutamine amidotransferase domain of CTP synthase mediates allosteric GTP activation of ...
|
|
22499
|
Lid L11 of the glutamine amidotransferase domain of CTP synthase mediates allosteric GTP activation of ...
|
|
22500
|
Identification of the sequence encoding N-acetylneuraminate-9-phosphate phosphatase
|
|
22501
|
Identification of the sequence encoding N-acetylneuraminate-9-phosphate phosphatase
|
|
22502
|
Identification of the sequence encoding N-acetylneuraminate-9-phosphate phosphatase
|
|
22503
|
Identification of the sequence encoding N-acetylneuraminate-9-phosphate phosphatase
|
|
22504
|
Identification of the sequence encoding N-acetylneuraminate-9-phosphate phosphatase
|
|
22505
|
Identification of the sequence encoding N-acetylneuraminate-9-phosphate phosphatase
|
|
22506
|
Identification of the sequence encoding N-acetylneuraminate-9-phosphate phosphatase
|
|
22507
|
Identification of the sequence encoding N-acetylneuraminate-9-phosphate phosphatase
|
|
22508
|
Identification of the sequence encoding N-acetylneuraminate-9-phosphate phosphatase
|
|
22509
|
Identification of the sequence encoding N-acetylneuraminate-9-phosphate phosphatase
|
|
22510
|
Identification of the sequence encoding N-acetylneuraminate-9-phosphate phosphatase
|
|
22511
|
Identification of the sequence encoding N-acetylneuraminate-9-phosphate phosphatase
|
|
22512
|
Identification of the sequence encoding N-acetylneuraminate-9-phosphate phosphatase
|
|
22513
|
Identification of the sequence encoding N-acetylneuraminate-9-phosphate phosphatase
|
|
22514
|
Identification of the sequence encoding N-acetylneuraminate-9-phosphate phosphatase
|
|
22515
|
Identification of the sequence encoding N-acetylneuraminate-9-phosphate phosphatase
|
|
22516
|
Identification of the sequence encoding N-acetylneuraminate-9-phosphate phosphatase
|
|
22517
|
Identification of the sequence encoding N-acetylneuraminate-9-phosphate phosphatase
|
|
22518
|
Identification of the sequence encoding N-acetylneuraminate-9-phosphate phosphatase
|
|
22519
|
Identification of the sequence encoding N-acetylneuraminate-9-phosphate phosphatase
|
|
22520
|
Identification of the sequence encoding N-acetylneuraminate-9-phosphate phosphatase
|
|
22521
|
Identification of the sequence encoding N-acetylneuraminate-9-phosphate phosphatase
|
|
22522
|
Identification of the sequence encoding N-acetylneuraminate-9-phosphate phosphatase
|
|
22523
|
Identification of the sequence encoding N-acetylneuraminate-9-phosphate phosphatase
|
|
22524
|
Identification of the sequence encoding N-acetylneuraminate-9-phosphate phosphatase
|
|
22525
|
Identification of the sequence encoding N-acetylneuraminate-9-phosphate phosphatase
|
|
22526
|
On the mechanism and control of the malonyl-CoA-dependent chain elongation of fatty acids. The ...
|
|
22527
|
On the mechanism and control of the malonyl-CoA-dependent chain elongation of fatty acids. The ...
|
|
22528
|
On the mechanism and control of the malonyl-CoA-dependent chain elongation of fatty acids. The ...
|
|
22529
|
On the mechanism and control of the malonyl-CoA-dependent chain elongation of fatty acids. The ...
|
|
22530
|
On the mechanism and control of the malonyl-CoA-dependent chain elongation of fatty acids. The ...
|
|
22531
|
On the mechanism and control of the malonyl-CoA-dependent chain elongation of fatty acids. The ...
|
|
22532
|
Use of six chimeric proteins to investigate the role of intramolecular interactions in determining the ...
|
|
22533
|
Use of six chimeric proteins to investigate the role of intramolecular interactions in determining the ...
|
|
22534
|
Use of six chimeric proteins to investigate the role of intramolecular interactions in determining the ...
|
|
22535
|
Use of six chimeric proteins to investigate the role of intramolecular interactions in determining the ...
|
|
22536
|
Use of six chimeric proteins to investigate the role of intramolecular interactions in determining the ...
|
|
22537
|
Use of six chimeric proteins to investigate the role of intramolecular interactions in determining the ...
|
|
22538
|
Use of six chimeric proteins to investigate the role of intramolecular interactions in determining the ...
|
|
22539
|
Use of six chimeric proteins to investigate the role of intramolecular interactions in determining the ...
|
|
22540
|
Use of six chimeric proteins to investigate the role of intramolecular interactions in determining the ...
|
|
22541
|
Use of six chimeric proteins to investigate the role of intramolecular interactions in determining the ...
|
|
22542
|
Use of six chimeric proteins to investigate the role of intramolecular interactions in determining the ...
|
|
22543
|
Use of six chimeric proteins to investigate the role of intramolecular interactions in determining the ...
|
|
22544
|
Use of six chimeric proteins to investigate the role of intramolecular interactions in determining the ...
|
|
22545
|
Use of six chimeric proteins to investigate the role of intramolecular interactions in determining the ...
|
|
22546
|
Use of six chimeric proteins to investigate the role of intramolecular interactions in determining the ...
|
|
22547
|
Use of six chimeric proteins to investigate the role of intramolecular interactions in determining the ...
|
|
22548
|
Use of six chimeric proteins to investigate the role of intramolecular interactions in determining the ...
|
|
22549
|
Use of six chimeric proteins to investigate the role of intramolecular interactions in determining the ...
|
|
22550
|
Use of six chimeric proteins to investigate the role of intramolecular interactions in determining the ...
|
|
22551
|
Use of six chimeric proteins to investigate the role of intramolecular interactions in determining the ...
|
|
22552
|
Use of six chimeric proteins to investigate the role of intramolecular interactions in determining the ...
|
|
22553
|
Use of six chimeric proteins to investigate the role of intramolecular interactions in determining the ...
|
|
22554
|
Use of six chimeric proteins to investigate the role of intramolecular interactions in determining the ...
|
|
22555
|
Use of six chimeric proteins to investigate the role of intramolecular interactions in determining the ...
|
|
22556
|
A single amino acid change (substitution of the conserved Glu-590 with alanine) in the C-terminal domain of ...
|
|
22557
|
A single amino acid change (substitution of the conserved Glu-590 with alanine) in the C-terminal domain of ...
|
|
22558
|
A single amino acid change (substitution of the conserved Glu-590 with alanine) in the C-terminal domain of ...
|
|
22559
|
A single amino acid change (substitution of the conserved Glu-590 with alanine) in the C-terminal domain of ...
|
|
22560
|
A single amino acid change (substitution of the conserved Glu-590 with alanine) in the C-terminal domain of ...
|
|
22561
|
A single amino acid change (substitution of the conserved Glu-590 with alanine) in the C-terminal domain of ...
|
|
22562
|
A single amino acid change (substitution of the conserved Glu-590 with alanine) in the C-terminal domain of ...
|
|
22563
|
A single amino acid change (substitution of the conserved Glu-590 with alanine) in the C-terminal domain of ...
|
|
22564
|
A single amino acid change (substitution of the conserved Glu-590 with alanine) in the C-terminal domain of ...
|
|
22565
|
A single amino acid change (substitution of the conserved Glu-590 with alanine) in the C-terminal domain of ...
|
|
22566
|
Identification by mutagenesis of conserved arginine and glutamate residues in the C-terminal domain of rat ...
|
|
22567
|
Identification by mutagenesis of conserved arginine and glutamate residues in the C-terminal domain of rat ...
|
|
22568
|
Identification by mutagenesis of conserved arginine and glutamate residues in the C-terminal domain of rat ...
|
|
22569
|
Identification by mutagenesis of conserved arginine and glutamate residues in the C-terminal domain of rat ...
|
|
22570
|
Identification by mutagenesis of conserved arginine and glutamate residues in the C-terminal domain of rat ...
|
|
22571
|
Identification by mutagenesis of conserved arginine and glutamate residues in the C-terminal domain of rat ...
|
|
22572
|
Identification by mutagenesis of conserved arginine and glutamate residues in the C-terminal domain of rat ...
|
|
22573
|
Identification by mutagenesis of conserved arginine and glutamate residues in the C-terminal domain of rat ...
|
|
22574
|
Identification by mutagenesis of conserved arginine and glutamate residues in the C-terminal domain of rat ...
|
|
22575
|
Identification by mutagenesis of conserved arginine and glutamate residues in the C-terminal domain of rat ...
|
|
22576
|
Identification by mutagenesis of conserved arginine and glutamate residues in the C-terminal domain of rat ...
|
|
22577
|
The extreme C terminus of rat liver carnitine palmitoyltransferase I is not involved in malonyl-CoA ...
|
|
22578
|
The extreme C terminus of rat liver carnitine palmitoyltransferase I is not involved in malonyl-CoA ...
|
|
22579
|
The extreme C terminus of rat liver carnitine palmitoyltransferase I is not involved in malonyl-CoA ...
|
|
22580
|
The extreme C terminus of rat liver carnitine palmitoyltransferase I is not involved in malonyl-CoA ...
|
|
22581
|
The extreme C terminus of rat liver carnitine palmitoyltransferase I is not involved in malonyl-CoA ...
|
|
22582
|
The extreme C terminus of rat liver carnitine palmitoyltransferase I is not involved in malonyl-CoA ...
|
|
22583
|
The extreme C terminus of rat liver carnitine palmitoyltransferase I is not involved in malonyl-CoA ...
|
|
22584
|
The extreme C terminus of rat liver carnitine palmitoyltransferase I is not involved in malonyl-CoA ...
|
|
22585
|
The extreme C terminus of rat liver carnitine palmitoyltransferase I is not involved in malonyl-CoA ...
|
|
22586
|
A single amino acid change (substitution of glutamate 3 with alanine) in the N-terminal region of rat liver ...
|
|
22587
|
A single amino acid change (substitution of glutamate 3 with alanine) in the N-terminal region of rat liver ...
|
|
22588
|
A single amino acid change (substitution of glutamate 3 with alanine) in the N-terminal region of rat liver ...
|
|
22589
|
A single amino acid change (substitution of glutamate 3 with alanine) in the N-terminal region of rat liver ...
|
|
22590
|
A single amino acid change (substitution of glutamate 3 with alanine) in the N-terminal region of rat liver ...
|
|
22591
|
A single amino acid change (substitution of glutamate 3 with alanine) in the N-terminal region of rat liver ...
|
|
22592
|
A single amino acid change (substitution of glutamate 3 with alanine) in the N-terminal region of rat liver ...
|
|
22593
|
A single amino acid change (substitution of glutamate 3 with alanine) in the N-terminal region of rat liver ...
|
|
22594
|
A single amino acid change (substitution of glutamate 3 with alanine) in the N-terminal region of rat liver ...
|
|
22595
|
A single amino acid change (substitution of glutamate 3 with alanine) in the N-terminal region of rat liver ...
|
|
22596
|
Carbamyl and acetyl phosphokinase activities of Streptococcus faecalis and Escherichia coli
|
|
22597
|
Carbamyl and acetyl phosphokinase activities of Streptococcus faecalis and Escherichia coli
|
|
22598
|
Carbamyl and acetyl phosphokinase activities of Streptococcus faecalis and Escherichia coli
|
|
22599
|
Carbamyl and acetyl phosphokinase activities of Streptococcus faecalis and Escherichia coli
|
|
22600
|
Carbamyl and acetyl phosphokinase activities of Streptococcus faecalis and Escherichia coli
|
|
22601
|
Carbamyl and acetyl phosphokinase activities of Streptococcus faecalis and Escherichia coli
|
|
22602
|
Carbamyl and acetyl phosphokinase activities of Streptococcus faecalis and Escherichia coli
|
|
22603
|
Carbamyl and acetyl phosphokinase activities of Streptococcus faecalis and Escherichia coli
|
|
22604
|
Carbamyl and acetyl phosphokinase activities of Streptococcus faecalis and Escherichia coli
|
|
22605
|
Carbamyl and acetyl phosphokinase activities of Streptococcus faecalis and Escherichia coli
|
|
22606
|
Carbamyl and acetyl phosphokinase activities of Streptococcus faecalis and Escherichia coli
|
|
22607
|
Carbamyl and acetyl phosphokinase activities of Streptococcus faecalis and Escherichia coli
|
|
22608
|
Carbamyl and acetyl phosphokinase activities of Streptococcus faecalis and Escherichia coli
|
|
22609
|
Purification and characterization of methylmalonate-semialdehyde dehydrogenase from rat liver. Identity to ...
|
|
22610
|
Purification and characterization of methylmalonate-semialdehyde dehydrogenase from rat liver. Identity to ...
|
|
22611
|
Purification and characterization of methylmalonate-semialdehyde dehydrogenase from rat liver. Identity to ...
|
|
22612
|
Purification and characterization of methylmalonate-semialdehyde dehydrogenase from rat liver. Identity to ...
|
|
22613
|
Purification and characterization of methylmalonate-semialdehyde dehydrogenase from rat liver. Identity to ...
|
|
22614
|
Investigations on pantothenic acid and its related compounds. X. Biochemical studies. 5. Purification and ...
|
|
22615
|
Investigations on pantothenic acid and its related compounds. X. Biochemical studies. 5. Purification and ...
|
|
22616
|
Investigations on pantothenic acid and its related compounds. X. Biochemical studies. 5. Purification and ...
|
|
22617
|
Purification and characterization of amino-acid N-choloyltransferase from human liver
|
|
22618
|
Purification and characterization of amino-acid N-choloyltransferase from human liver
|
|
22619
|
Purification and characterization of amino-acid N-choloyltransferase from human liver
|
|
22620
|
Purification and characterization of amino-acid N-choloyltransferase from human liver
|
|
22621
|
Mouse mitochondrial aldehyde dehydrogenase isozymes: purification and molecular properties
|
|
22622
|
Mouse mitochondrial aldehyde dehydrogenase isozymes: purification and molecular properties
|
|
22623
|
Mouse mitochondrial aldehyde dehydrogenase isozymes: purification and molecular properties
|
|
22624
|
Mouse mitochondrial aldehyde dehydrogenase isozymes: purification and molecular properties
|
|
22625
|
Mouse mitochondrial aldehyde dehydrogenase isozymes: purification and molecular properties
|
|
22626
|
Mouse mitochondrial aldehyde dehydrogenase isozymes: purification and molecular properties
|
|
22627
|
Mouse mitochondrial aldehyde dehydrogenase isozymes: purification and molecular properties
|
|
22628
|
Mouse mitochondrial aldehyde dehydrogenase isozymes: purification and molecular properties
|
|
22629
|
Mouse mitochondrial aldehyde dehydrogenase isozymes: purification and molecular properties
|
|
22630
|
Mouse mitochondrial aldehyde dehydrogenase isozymes: purification and molecular properties
|
|
22631
|
Kinetic properties of human pancreatic carboxylesterase
|
|
22632
|
Kinetic properties of human pancreatic carboxylesterase
|
|
22633
|
Kinetic properties of human pancreatic carboxylesterase
|
|
22634
|
Kinetic properties of human pancreatic carboxylesterase
|
|
22635
|
Kinetic properties of human pancreatic carboxylesterase
|
|
22636
|
Kinetic properties of human pancreatic carboxylesterase
|
|
22637
|
Kinetic properties of human pancreatic carboxylesterase
|
|
22638
|
Phosphoryl exchange reaction catalyzed by enzyme I of the bacterial phosphoenolpyruvate: sugar ...
|
|
22639
|
Phosphoryl exchange reaction catalyzed by enzyme I of the bacterial phosphoenolpyruvate: sugar ...
|
|
22640
|
Phosphoryl exchange reaction catalyzed by enzyme I of the bacterial phosphoenolpyruvate: sugar ...
|
|
22641
|
Phosphoryl exchange reaction catalyzed by enzyme I of the bacterial phosphoenolpyruvate: sugar ...
|
|
22642
|
Phosphoryl exchange reaction catalyzed by enzyme I of the bacterial phosphoenolpyruvate: sugar ...
|
|
22643
|
Phosphoryl exchange reaction catalyzed by enzyme I of the bacterial phosphoenolpyruvate: sugar ...
|
|
22644
|
Phosphoryl exchange reaction catalyzed by enzyme I of the bacterial phosphoenolpyruvate: sugar ...
|
|
22645
|
Phosphoryl exchange reaction catalyzed by enzyme I of the bacterial phosphoenolpyruvate: sugar ...
|
|
22646
|
Phosphoryl exchange reaction catalyzed by enzyme I of the bacterial phosphoenolpyruvate: sugar ...
|
|
22647
|
Phosphoryl exchange reaction catalyzed by enzyme I of the bacterial phosphoenolpyruvate: sugar ...
|
|
22648
|
Biochemical characterization of cytosolic fructose-1,6-bisphosphatase from apple (Malus domestica) leaves
|
|
22649
|
Biochemical characterization of cytosolic fructose-1,6-bisphosphatase from apple (Malus domestica) leaves
|
|
22650
|
Biochemical characterization of cytosolic fructose-1,6-bisphosphatase from apple (Malus domestica) leaves
|
|
22651
|
Biochemical characterization of cytosolic fructose-1,6-bisphosphatase from apple (Malus domestica) leaves
|
|
22652
|
Biochemical characterization of cytosolic fructose-1,6-bisphosphatase from apple (Malus domestica) leaves
|
|
22653
|
Characterization of active-site mutants of Schizosaccharomyces pombe phosphoglycerate mutase. Elucidation of ...
|
|
22654
|
Characterization of active-site mutants of Schizosaccharomyces pombe phosphoglycerate mutase. Elucidation of ...
|
|
22655
|
Characterization of active-site mutants of Schizosaccharomyces pombe phosphoglycerate mutase. Elucidation of ...
|
|
22656
|
Characterization of active-site mutants of Schizosaccharomyces pombe phosphoglycerate mutase. Elucidation of ...
|
|
22657
|
Characterization of active-site mutants of Schizosaccharomyces pombe phosphoglycerate mutase. Elucidation of ...
|
|
22658
|
Characterization of active-site mutants of Schizosaccharomyces pombe phosphoglycerate mutase. Elucidation of ...
|
|
22659
|
Characterization of active-site mutants of Schizosaccharomyces pombe phosphoglycerate mutase. Elucidation of ...
|
|
22660
|
Characterization of active-site mutants of Schizosaccharomyces pombe phosphoglycerate mutase. Elucidation of ...
|
|
22661
|
Characterization of active-site mutants of Schizosaccharomyces pombe phosphoglycerate mutase. Elucidation of ...
|
|
22662
|
Characterization of active-site mutants of Schizosaccharomyces pombe phosphoglycerate mutase. Elucidation of ...
|
|
22663
|
Characterization of active-site mutants of Schizosaccharomyces pombe phosphoglycerate mutase. Elucidation of ...
|
|
22664
|
Characterization of active-site mutants of Schizosaccharomyces pombe phosphoglycerate mutase. Elucidation of ...
|
|
22665
|
Characterization of active-site mutants of Schizosaccharomyces pombe phosphoglycerate mutase. Elucidation of ...
|
|
22666
|
Characterization of active-site mutants of Schizosaccharomyces pombe phosphoglycerate mutase. Elucidation of ...
|
|
22667
|
Shifting the NAD/NADP preference in class 3 aldehyde dehydrogenase
|
|
22668
|
Shifting the NAD/NADP preference in class 3 aldehyde dehydrogenase
|
|
22669
|
Shifting the NAD/NADP preference in class 3 aldehyde dehydrogenase
|
|
22670
|
Shifting the NAD/NADP preference in class 3 aldehyde dehydrogenase
|
|
22671
|
Shifting the NAD/NADP preference in class 3 aldehyde dehydrogenase
|
|
22672
|
Shifting the NAD/NADP preference in class 3 aldehyde dehydrogenase
|
|
22673
|
Shifting the NAD/NADP preference in class 3 aldehyde dehydrogenase
|
|
22674
|
Shifting the NAD/NADP preference in class 3 aldehyde dehydrogenase
|
|
22675
|
Shifting the NAD/NADP preference in class 3 aldehyde dehydrogenase
|
|
22676
|
Shifting the NAD/NADP preference in class 3 aldehyde dehydrogenase
|
|
22677
|
Shifting the NAD/NADP preference in class 3 aldehyde dehydrogenase
|
|
22678
|
Shifting the NAD/NADP preference in class 3 aldehyde dehydrogenase
|
|
22679
|
Shifting the NAD/NADP preference in class 3 aldehyde dehydrogenase
|
|
22680
|
Shifting the NAD/NADP preference in class 3 aldehyde dehydrogenase
|
|
22681
|
Shifting the NAD/NADP preference in class 3 aldehyde dehydrogenase
|
|
22682
|
Shifting the NAD/NADP preference in class 3 aldehyde dehydrogenase
|
|
22683
|
Shifting the NAD/NADP preference in class 3 aldehyde dehydrogenase
|
|
22684
|
Shifting the NAD/NADP preference in class 3 aldehyde dehydrogenase
|
|
22685
|
Shifting the NAD/NADP preference in class 3 aldehyde dehydrogenase
|
|
22686
|
Shifting the NAD/NADP preference in class 3 aldehyde dehydrogenase
|
|
22687
|
Shifting the NAD/NADP preference in class 3 aldehyde dehydrogenase
|
|
22688
|
Shifting the NAD/NADP preference in class 3 aldehyde dehydrogenase
|
|
22689
|
Shifting the NAD/NADP preference in class 3 aldehyde dehydrogenase
|
|
22690
|
Shifting the NAD/NADP preference in class 3 aldehyde dehydrogenase
|
|
22691
|
Shifting the NAD/NADP preference in class 3 aldehyde dehydrogenase
|
|
22692
|
Shifting the NAD/NADP preference in class 3 aldehyde dehydrogenase
|
|
22693
|
Shifting the NAD/NADP preference in class 3 aldehyde dehydrogenase
|
|
22694
|
Shifting the NAD/NADP preference in class 3 aldehyde dehydrogenase
|
|
22695
|
Shifting the NAD/NADP preference in class 3 aldehyde dehydrogenase
|
|
22696
|
Shifting the NAD/NADP preference in class 3 aldehyde dehydrogenase
|
|
22697
|
Shifting the NAD/NADP preference in class 3 aldehyde dehydrogenase
|
|
22698
|
Shifting the NAD/NADP preference in class 3 aldehyde dehydrogenase
|
|
22699
|
Shifting the NAD/NADP preference in class 3 aldehyde dehydrogenase
|
|
22700
|
Shifting the NAD/NADP preference in class 3 aldehyde dehydrogenase
|
|
22701
|
Human hepatic methionine biosynthesis. Purification and characterization of betaine:homocysteine ...
|
|
22702
|
Human hepatic methionine biosynthesis. Purification and characterization of betaine:homocysteine ...
|
|
22703
|
Human hepatic methionine biosynthesis. Purification and characterization of betaine:homocysteine ...
|
|
22704
|
Human hepatic methionine biosynthesis. Purification and characterization of betaine:homocysteine ...
|
|
22705
|
Transiently reduced activity of carbamyl phosphate synthetase and ornithine transcarbamylase in liver of ...
|
|
22706
|
Transiently reduced activity of carbamyl phosphate synthetase and ornithine transcarbamylase in liver of ...
|
|
22707
|
Transiently reduced activity of carbamyl phosphate synthetase and ornithine transcarbamylase in liver of ...
|
|
22708
|
The anaerobic (class III) ribonucleotide reductase from Lactococcus lactis. Catalytic properties and ...
|
|
22709
|
The anaerobic (class III) ribonucleotide reductase from Lactococcus lactis. Catalytic properties and ...
|
|
22710
|
The anaerobic (class III) ribonucleotide reductase from Lactococcus lactis. Catalytic properties and ...
|
|
22711
|
The anaerobic (class III) ribonucleotide reductase from Lactococcus lactis. Catalytic properties and ...
|
|
22712
|
The anaerobic (class III) ribonucleotide reductase from Lactococcus lactis. Catalytic properties and ...
|
|
22713
|
The anaerobic (class III) ribonucleotide reductase from Lactococcus lactis. Catalytic properties and ...
|
|
22714
|
The anaerobic (class III) ribonucleotide reductase from Lactococcus lactis. Catalytic properties and ...
|
|
22715
|
The anaerobic (class III) ribonucleotide reductase from Lactococcus lactis. Catalytic properties and ...
|
|
22716
|
The anaerobic (class III) ribonucleotide reductase from Lactococcus lactis. Catalytic properties and ...
|
|
22717
|
An active site homology model of phenylalanine ammonia-lyase from Petroselinum crispum
|
|
22718
|
An active site homology model of phenylalanine ammonia-lyase from Petroselinum crispum
|
|
22719
|
An active site homology model of phenylalanine ammonia-lyase from Petroselinum crispum
|
|
22720
|
An active site homology model of phenylalanine ammonia-lyase from Petroselinum crispum
|
|
22721
|
An active site homology model of phenylalanine ammonia-lyase from Petroselinum crispum
|
|
22722
|
An active site homology model of phenylalanine ammonia-lyase from Petroselinum crispum
|
|
22723
|
An active site homology model of phenylalanine ammonia-lyase from Petroselinum crispum
|
|
22724
|
An active site homology model of phenylalanine ammonia-lyase from Petroselinum crispum
|
|
22725
|
An active site homology model of phenylalanine ammonia-lyase from Petroselinum crispum
|
|
22726
|
An active site homology model of phenylalanine ammonia-lyase from Petroselinum crispum
|
|
22727
|
An active site homology model of phenylalanine ammonia-lyase from Petroselinum crispum
|
|
22728
|
An active site homology model of phenylalanine ammonia-lyase from Petroselinum crispum
|
|
22729
|
Structural and functional characterization of rabbit and human L-gulonate 3-dehydrogenase
|
|
22730
|
Structural and functional characterization of rabbit and human L-gulonate 3-dehydrogenase
|
|
22731
|
Structural and functional characterization of rabbit and human L-gulonate 3-dehydrogenase
|
|
22732
|
Structural and functional characterization of rabbit and human L-gulonate 3-dehydrogenase
|
|
22733
|
Structural and functional characterization of rabbit and human L-gulonate 3-dehydrogenase
|
|
22734
|
Structural and functional characterization of rabbit and human L-gulonate 3-dehydrogenase
|
|
22735
|
Structural and functional characterization of rabbit and human L-gulonate 3-dehydrogenase
|
|
22736
|
Structural and functional characterization of rabbit and human L-gulonate 3-dehydrogenase
|
|
22737
|
Structural and functional characterization of rabbit and human L-gulonate 3-dehydrogenase
|
|
22738
|
Structural and functional characterization of rabbit and human L-gulonate 3-dehydrogenase
|
|
22739
|
Structural and functional characterization of rabbit and human L-gulonate 3-dehydrogenase
|
|
22740
|
Structural and functional characterization of rabbit and human L-gulonate 3-dehydrogenase
|
|
22741
|
Structural and functional characterization of rabbit and human L-gulonate 3-dehydrogenase
|
|
22742
|
Structural and functional characterization of rabbit and human L-gulonate 3-dehydrogenase
|
|
22743
|
Structural and functional characterization of rabbit and human L-gulonate 3-dehydrogenase
|
|
22744
|
Structural and functional characterization of rabbit and human L-gulonate 3-dehydrogenase
|
|
22745
|
Structural and functional characterization of rabbit and human L-gulonate 3-dehydrogenase
|
|
22746
|
Structural and functional characterization of rabbit and human L-gulonate 3-dehydrogenase
|
|
22747
|
Structural and functional characterization of rabbit and human L-gulonate 3-dehydrogenase
|
|
22748
|
Structural and functional characterization of rabbit and human L-gulonate 3-dehydrogenase
|
|
22749
|
Structural and functional characterization of rabbit and human L-gulonate 3-dehydrogenase
|
|
22750
|
Structural and functional characterization of rabbit and human L-gulonate 3-dehydrogenase
|
|
22751
|
Structural and functional characterization of rabbit and human L-gulonate 3-dehydrogenase
|
|
22752
|
Structural and functional characterization of rabbit and human L-gulonate 3-dehydrogenase
|
|
22753
|
Structural and functional characterization of rabbit and human L-gulonate 3-dehydrogenase
|
|
22754
|
Structural and functional characterization of rabbit and human L-gulonate 3-dehydrogenase
|
|
22755
|
Structural and functional characterization of rabbit and human L-gulonate 3-dehydrogenase
|
|
22756
|
Structural and functional characterization of rabbit and human L-gulonate 3-dehydrogenase
|
|
22757
|
Structural and functional characterization of rabbit and human L-gulonate 3-dehydrogenase
|
|
22758
|
Structural and functional characterization of rabbit and human L-gulonate 3-dehydrogenase
|
|
22759
|
Structural and functional characterization of rabbit and human L-gulonate 3-dehydrogenase
|
|
22760
|
Structural and functional characterization of rabbit and human L-gulonate 3-dehydrogenase
|
|
22761
|
Structural and functional characterization of rabbit and human L-gulonate 3-dehydrogenase
|
|
22762
|
Structural and functional characterization of rabbit and human L-gulonate 3-dehydrogenase
|
|
22763
|
Structural and functional characterization of rabbit and human L-gulonate 3-dehydrogenase
|
|
22764
|
Structural and functional characterization of rabbit and human L-gulonate 3-dehydrogenase
|
|
22765
|
Structural and functional characterization of rabbit and human L-gulonate 3-dehydrogenase
|
|
22766
|
Structural and functional characterization of rabbit and human L-gulonate 3-dehydrogenase
|
|
22767
|
Structural and functional characterization of rabbit and human L-gulonate 3-dehydrogenase
|
|
22768
|
Structural and functional characterization of rabbit and human L-gulonate 3-dehydrogenase
|
|
22769
|
Structural and functional characterization of rabbit and human L-gulonate 3-dehydrogenase
|
|
22770
|
Structural and functional characterization of rabbit and human L-gulonate 3-dehydrogenase
|
|
22771
|
Structural and functional characterization of rabbit and human L-gulonate 3-dehydrogenase
|
|
22772
|
Structural and functional characterization of rabbit and human L-gulonate 3-dehydrogenase
|
|
22773
|
Structural and functional characterization of rabbit and human L-gulonate 3-dehydrogenase
|
|
22774
|
Structural and functional characterization of rabbit and human L-gulonate 3-dehydrogenase
|
|
22775
|
Structural and functional characterization of rabbit and human L-gulonate 3-dehydrogenase
|
|
22776
|
Structural and functional characterization of rabbit and human L-gulonate 3-dehydrogenase
|
|
22777
|
Structural and functional characterization of rabbit and human L-gulonate 3-dehydrogenase
|
|
22778
|
Structural and functional characterization of rabbit and human L-gulonate 3-dehydrogenase
|
|
22779
|
Structural and functional characterization of rabbit and human L-gulonate 3-dehydrogenase
|
|
22780
|
Structural and functional characterization of rabbit and human L-gulonate 3-dehydrogenase
|
|
22781
|
Structural and functional characterization of rabbit and human L-gulonate 3-dehydrogenase
|
|
22782
|
Structural and functional characterization of rabbit and human L-gulonate 3-dehydrogenase
|
|
22783
|
Structural and functional characterization of rabbit and human L-gulonate 3-dehydrogenase
|
|
22784
|
Structural and functional characterization of rabbit and human L-gulonate 3-dehydrogenase
|
|
22785
|
Structural and functional characterization of rabbit and human L-gulonate 3-dehydrogenase
|
|
22786
|
Structural and functional characterization of rabbit and human L-gulonate 3-dehydrogenase
|
|
22787
|
Structural and functional characterization of rabbit and human L-gulonate 3-dehydrogenase
|
|
22788
|
Kinetic studies on endo-beta-galactosidase by a novel colorimetric assay and synthesis of ...
|
|
22789
|
Kinetic studies on endo-beta-galactosidase by a novel colorimetric assay and synthesis of ...
|
|
22790
|
Kinetic studies on endo-beta-galactosidase by a novel colorimetric assay and synthesis of ...
|
|
22791
|
Kinetic studies on endo-beta-galactosidase by a novel colorimetric assay and synthesis of ...
|
|
22792
|
Kinetic studies on endo-beta-galactosidase by a novel colorimetric assay and synthesis of ...
|
|
22793
|
Kinetic studies on endo-beta-galactosidase by a novel colorimetric assay and synthesis of ...
|
|
22794
|
Kinetic studies on endo-beta-galactosidase by a novel colorimetric assay and synthesis of ...
|
|
22795
|
Kinetic studies on endo-beta-galactosidase by a novel colorimetric assay and synthesis of ...
|
|
22796
|
Kinetic studies on endo-beta-galactosidase by a novel colorimetric assay and synthesis of ...
|
|
22797
|
Effect of ATP on glucose-6-phosphate isomerase from Bacillus caldotenax
|
|
22798
|
Effect of ATP on glucose-6-phosphate isomerase from Bacillus caldotenax
|
|
22799
|
Effect of ATP on glucose-6-phosphate isomerase from Bacillus caldotenax
|
|
22800
|
Effect of ATP on glucose-6-phosphate isomerase from Bacillus caldotenax
|
|
22801
|
Effect of ATP on glucose-6-phosphate isomerase from Bacillus caldotenax
|
|
22802
|
Effect of ATP on glucose-6-phosphate isomerase from Bacillus caldotenax
|
|
22803
|
Effect of ATP on glucose-6-phosphate isomerase from Bacillus caldotenax
|
|
22804
|
Effect of ATP on glucose-6-phosphate isomerase from Bacillus caldotenax
|
|
22805
|
Role of L-citrulline transport in nitric oxide synthesis in rat aortic smooth muscle cells activated with LPS ...
|
|
22806
|
Role of L-citrulline transport in nitric oxide synthesis in rat aortic smooth muscle cells activated with LPS ...
|
|
22807
|
Role of L-citrulline transport in nitric oxide synthesis in rat aortic smooth muscle cells activated with LPS ...
|
|
22808
|
Role of L-citrulline transport in nitric oxide synthesis in rat aortic smooth muscle cells activated with LPS ...
|
|
22809
|
Role of L-citrulline transport in nitric oxide synthesis in rat aortic smooth muscle cells activated with LPS ...
|
|
22810
|
Mechanistic studies of reaction coupling in Glu-tRNAGln amidotransferase
|
|
22811
|
Mechanistic studies of reaction coupling in Glu-tRNAGln amidotransferase
|
|
22812
|
Mechanistic studies of reaction coupling in Glu-tRNAGln amidotransferase
|
|
22813
|
Mechanistic studies of reaction coupling in Glu-tRNAGln amidotransferase
|
|
22814
|
Mechanistic studies of reaction coupling in Glu-tRNAGln amidotransferase
|
|
22815
|
Mechanistic studies of reaction coupling in Glu-tRNAGln amidotransferase
|
|
22816
|
Mechanistic studies of reaction coupling in Glu-tRNAGln amidotransferase
|
|
22817
|
Mechanistic studies of reaction coupling in Glu-tRNAGln amidotransferase
|
|
22818
|
Mechanistic studies of reaction coupling in Glu-tRNAGln amidotransferase
|
|
22819
|
Mechanistic studies of reaction coupling in Glu-tRNAGln amidotransferase
|
|
22820
|
Mechanistic studies of reaction coupling in Glu-tRNAGln amidotransferase
|
|
22821
|
Mechanistic studies of reaction coupling in Glu-tRNAGln amidotransferase
|
|
22822
|
Mechanistic studies of reaction coupling in Glu-tRNAGln amidotransferase
|
|
22823
|
Mechanistic studies of reaction coupling in Glu-tRNAGln amidotransferase
|
|
22824
|
Mechanistic studies of reaction coupling in Glu-tRNAGln amidotransferase
|
|
22825
|
Mechanistic studies of reaction coupling in Glu-tRNAGln amidotransferase
|
|
22826
|
Kinetic parameters and tissue distribution of 5-oxo-L-prolinase determined by a fluorimetric assay
|
|
22827
|
Kinetic parameters and tissue distribution of 5-oxo-L-prolinase determined by a fluorimetric assay
|
|
22828
|
Kinetic parameters and tissue distribution of 5-oxo-L-prolinase determined by a fluorimetric assay
|
|
22829
|
Kinetic parameters and tissue distribution of 5-oxo-L-prolinase determined by a fluorimetric assay
|
|
22830
|
Kinetic parameters and tissue distribution of 5-oxo-L-prolinase determined by a fluorimetric assay
|
|
22831
|
Kinetic parameters and tissue distribution of 5-oxo-L-prolinase determined by a fluorimetric assay
|
|
22832
|
Kinetic parameters and tissue distribution of 5-oxo-L-prolinase determined by a fluorimetric assay
|
|
22833
|
Analysis of the kinetic mechanism of haloalkane conjugation by mammalian theta-class glutathione transferases
|
|
22834
|
Analysis of the kinetic mechanism of haloalkane conjugation by mammalian theta-class glutathione transferases
|
|
22835
|
Analysis of the kinetic mechanism of haloalkane conjugation by mammalian theta-class glutathione transferases
|
|
22836
|
Analysis of the kinetic mechanism of haloalkane conjugation by mammalian theta-class glutathione transferases
|
|
22837
|
Analysis of the kinetic mechanism of haloalkane conjugation by mammalian theta-class glutathione transferases
|
|
22838
|
Analysis of the kinetic mechanism of haloalkane conjugation by mammalian theta-class glutathione transferases
|
|
22839
|
Analysis of the kinetic mechanism of haloalkane conjugation by mammalian theta-class glutathione transferases
|
|
22840
|
The Enhanced Affinity for Thiolate Anion and Activation of Enzyme-bound Glutathione Is Governed by an Arginine ...
|
|
22841
|
The Enhanced Affinity for Thiolate Anion and Activation of Enzyme-bound Glutathione Is Governed by an Arginine ...
|
|
22842
|
The Enhanced Affinity for Thiolate Anion and Activation of Enzyme-bound Glutathione Is Governed by an Arginine ...
|
|
22843
|
The Enhanced Affinity for Thiolate Anion and Activation of Enzyme-bound Glutathione Is Governed by an Arginine ...
|
|
22844
|
The Enhanced Affinity for Thiolate Anion and Activation of Enzyme-bound Glutathione Is Governed by an Arginine ...
|
|
22845
|
The Enhanced Affinity for Thiolate Anion and Activation of Enzyme-bound Glutathione Is Governed by an Arginine ...
|
|
22846
|
The Enhanced Affinity for Thiolate Anion and Activation of Enzyme-bound Glutathione Is Governed by an Arginine ...
|
|
22847
|
The Enhanced Affinity for Thiolate Anion and Activation of Enzyme-bound Glutathione Is Governed by an Arginine ...
|
|
22848
|
The Enhanced Affinity for Thiolate Anion and Activation of Enzyme-bound Glutathione Is Governed by an Arginine ...
|
|
22849
|
The Enhanced Affinity for Thiolate Anion and Activation of Enzyme-bound Glutathione Is Governed by an Arginine ...
|
|
22850
|
The Enhanced Affinity for Thiolate Anion and Activation of Enzyme-bound Glutathione Is Governed by an Arginine ...
|
|
22851
|
The Enhanced Affinity for Thiolate Anion and Activation of Enzyme-bound Glutathione Is Governed by an Arginine ...
|
|
22852
|
The Enhanced Affinity for Thiolate Anion and Activation of Enzyme-bound Glutathione Is Governed by an Arginine ...
|
|
22853
|
The Enhanced Affinity for Thiolate Anion and Activation of Enzyme-bound Glutathione Is Governed by an Arginine ...
|
|
22854
|
The Enhanced Affinity for Thiolate Anion and Activation of Enzyme-bound Glutathione Is Governed by an Arginine ...
|
|
22855
|
The Enhanced Affinity for Thiolate Anion and Activation of Enzyme-bound Glutathione Is Governed by an Arginine ...
|
|
22856
|
Purification and characterisation of glyoxalase II from human red blood cells
|
|
22857
|
Purification and characterisation of glyoxalase II from human red blood cells
|
|
22858
|
Purification and characterisation of glyoxalase II from human red blood cells
|
|
22859
|
Purification and characterisation of glyoxalase II from human red blood cells
|
|
22860
|
Purification and characterisation of glyoxalase II from human red blood cells
|
|
22861
|
Purification and characterisation of glyoxalase II from human red blood cells
|
|
22862
|
Molecular cloning, heterologous expression, and characterization of human glyoxalase II
|
|
22863
|
Molecular cloning, heterologous expression, and characterization of human glyoxalase II
|
|
22864
|
Molecular cloning, heterologous expression, and characterization of human glyoxalase II
|
|
22865
|
Molecular cloning, heterologous expression, and characterization of human glyoxalase II
|
|
22866
|
Reduction of trioses by NADPH-dependent aldo-keto reductases. Aldose reductase, methylglyoxal, and diabetic ...
|
|
22867
|
Reduction of trioses by NADPH-dependent aldo-keto reductases. Aldose reductase, methylglyoxal, and diabetic ...
|
|
22868
|
Reduction of trioses by NADPH-dependent aldo-keto reductases. Aldose reductase, methylglyoxal, and diabetic ...
|
|
22869
|
Reduction of trioses by NADPH-dependent aldo-keto reductases. Aldose reductase, methylglyoxal, and diabetic ...
|
|
22870
|
Reduction of trioses by NADPH-dependent aldo-keto reductases. Aldose reductase, methylglyoxal, and diabetic ...
|
|
22871
|
Reduction of trioses by NADPH-dependent aldo-keto reductases. Aldose reductase, methylglyoxal, and diabetic ...
|
|
22872
|
Reduction of trioses by NADPH-dependent aldo-keto reductases. Aldose reductase, methylglyoxal, and diabetic ...
|
|
22873
|
Reduction of trioses by NADPH-dependent aldo-keto reductases. Aldose reductase, methylglyoxal, and diabetic ...
|
|
22874
|
Reduction of trioses by NADPH-dependent aldo-keto reductases. Aldose reductase, methylglyoxal, and diabetic ...
|
|
22875
|
Reduction of trioses by NADPH-dependent aldo-keto reductases. Aldose reductase, methylglyoxal, and diabetic ...
|
|
22876
|
Reduction of trioses by NADPH-dependent aldo-keto reductases. Aldose reductase, methylglyoxal, and diabetic ...
|
|
22877
|
Reduction of trioses by NADPH-dependent aldo-keto reductases. Aldose reductase, methylglyoxal, and diabetic ...
|
|
22878
|
Reduction of trioses by NADPH-dependent aldo-keto reductases. Aldose reductase, methylglyoxal, and diabetic ...
|
|
22879
|
Reduction of trioses by NADPH-dependent aldo-keto reductases. Aldose reductase, methylglyoxal, and diabetic ...
|
|
22880
|
Reduction of trioses by NADPH-dependent aldo-keto reductases. Aldose reductase, methylglyoxal, and diabetic ...
|
|
22881
|
Reduction of trioses by NADPH-dependent aldo-keto reductases. Aldose reductase, methylglyoxal, and diabetic ...
|
|
22882
|
Reduction of trioses by NADPH-dependent aldo-keto reductases. Aldose reductase, methylglyoxal, and diabetic ...
|
|
22883
|
Reduction of trioses by NADPH-dependent aldo-keto reductases. Aldose reductase, methylglyoxal, and diabetic ...
|
|
22884
|
Reduction of trioses by NADPH-dependent aldo-keto reductases. Aldose reductase, methylglyoxal, and diabetic ...
|
|
22885
|
Reduction of trioses by NADPH-dependent aldo-keto reductases. Aldose reductase, methylglyoxal, and diabetic ...
|
|
22886
|
Reduction of trioses by NADPH-dependent aldo-keto reductases. Aldose reductase, methylglyoxal, and diabetic ...
|
|
22887
|
Purification and characterization of human-brain aldose reductase
|
|
22888
|
Purification and characterization of human-brain aldose reductase
|
|
22889
|
Purification and characterization of human-brain aldose reductase
|
|
22890
|
Purification and characterization of human-brain aldose reductase
|
|
22891
|
Purification and characterization of human-brain aldose reductase
|
|
22892
|
Purification and characterization of human-brain aldose reductase
|
|
22893
|
Purification and characterization of human-brain aldose reductase
|
|
22894
|
Purification and characterization of human-brain aldose reductase
|
|
22895
|
Purification and characterization of human-brain aldose reductase
|
|
22896
|
Purification and characterization of human-brain aldose reductase
|
|
22897
|
Purification and characterization of human-brain aldose reductase
|
|
22898
|
Purification and characterization of human-brain aldose reductase
|
|
22899
|
Purification and characterization of human-brain aldose reductase
|
|
22900
|
Purification and characterization of human-brain aldose reductase
|
|
22901
|
Purification and characterization of human-brain aldose reductase
|
|
22902
|
Purification and characterization of human-brain aldose reductase
|
|
22903
|
Purification and characterization of human-brain aldose reductase
|
|
22904
|
Purification and characterization of human-brain aldose reductase
|
|
22905
|
Purification and characterization of human-brain aldose reductase
|
|
22906
|
Purification and characterization of human-brain aldose reductase
|
|
22907
|
Purification and characterization of human-brain aldose reductase
|
|
22908
|
Purification and characterization of human-brain aldose reductase
|
|
22909
|
A nudix enzyme removes pyrophosphate from dihydroneopterin triphosphate in the folate synthesis pathway of ...
|
|
22910
|
A nudix enzyme removes pyrophosphate from dihydroneopterin triphosphate in the folate synthesis pathway of ...
|
|
22911
|
A nudix enzyme removes pyrophosphate from dihydroneopterin triphosphate in the folate synthesis pathway of ...
|
|
22912
|
A nudix enzyme removes pyrophosphate from dihydroneopterin triphosphate in the folate synthesis pathway of ...
|
|
22913
|
A nudix enzyme removes pyrophosphate from dihydroneopterin triphosphate in the folate synthesis pathway of ...
|
|
22914
|
A nudix enzyme removes pyrophosphate from dihydroneopterin triphosphate in the folate synthesis pathway of ...
|
|
22915
|
A nudix enzyme removes pyrophosphate from dihydroneopterin triphosphate in the folate synthesis pathway of ...
|
|
22916
|
A nudix enzyme removes pyrophosphate from dihydroneopterin triphosphate in the folate synthesis pathway of ...
|
|
22917
|
A nudix enzyme removes pyrophosphate from dihydroneopterin triphosphate in the folate synthesis pathway of ...
|
|
22918
|
A nudix enzyme removes pyrophosphate from dihydroneopterin triphosphate in the folate synthesis pathway of ...
|
|
22919
|
A nudix enzyme removes pyrophosphate from dihydroneopterin triphosphate in the folate synthesis pathway of ...
|
|
22920
|
Properties of two sugar phosphate phosphatases from Streptococcus bovis and their potential involvement in ...
|
|
22921
|
Properties of two sugar phosphate phosphatases from Streptococcus bovis and their potential involvement in ...
|
|
22922
|
Properties of two sugar phosphate phosphatases from Streptococcus bovis and their potential involvement in ...
|
|
22923
|
Properties of two sugar phosphate phosphatases from Streptococcus bovis and their potential involvement in ...
|
|
22924
|
Choline kinase and ethanolamine kinase are separate, soluble enzymes in rat liver
|
|
22925
|
Choline kinase and ethanolamine kinase are separate, soluble enzymes in rat liver
|
|
22926
|
Choline kinase and ethanolamine kinase are separate, soluble enzymes in rat liver
|
|
22927
|
Choline kinase and ethanolamine kinase are separate, soluble enzymes in rat liver
|
|
22928
|
Isolation of glyoxalase II from bovine liver mitochondria
|
|
22929
|
Isolation of glyoxalase II from bovine liver mitochondria
|
|
22930
|
Isolation of glyoxalase II from bovine liver mitochondria
|
|
22931
|
Isolation of glyoxalase II from bovine liver mitochondria
|
|
22932
|
Functionally accepted insertions of proteins within protein domains
|
|
22933
|
Functionally accepted insertions of proteins within protein domains
|
|
22934
|
Functionally accepted insertions of proteins within protein domains
|
|
22935
|
Functionally accepted insertions of proteins within protein domains
|
|
22936
|
Functionally accepted insertions of proteins within protein domains
|
|
22937
|
Functionally accepted insertions of proteins within protein domains
|
|
22938
|
Functionally accepted insertions of proteins within protein domains
|
|
22939
|
Functionally accepted insertions of proteins within protein domains
|
|
22940
|
Functionally accepted insertions of proteins within protein domains
|
|
22941
|
Functionally accepted insertions of proteins within protein domains
|
|
22942
|
Functionally accepted insertions of proteins within protein domains
|
|
22943
|
Functionally accepted insertions of proteins within protein domains
|
|
22944
|
Functionally accepted insertions of proteins within protein domains
|
|
22945
|
Functionally accepted insertions of proteins within protein domains
|
|
22946
|
Functionally accepted insertions of proteins within protein domains
|
|
22947
|
Functionally accepted insertions of proteins within protein domains
|
|
22948
|
Functionally accepted insertions of proteins within protein domains
|
|
22949
|
Isolation and overexpression of a gene encoding an extracellular beta-(1,3-1,4)-glucanase from Streptococcus ...
|
|
22950
|
Isolation and overexpression of a gene encoding an extracellular beta-(1,3-1,4)-glucanase from Streptococcus ...
|
|
22951
|
Isolation and overexpression of a gene encoding an extracellular beta-(1,3-1,4)-glucanase from Streptococcus ...
|
|
22952
|
Determinants of the dual cofactor specificity and substrate cooperativity of the human mitochondrial ...
|
|
22953
|
Determinants of the dual cofactor specificity and substrate cooperativity of the human mitochondrial ...
|
|
22954
|
Determinants of the dual cofactor specificity and substrate cooperativity of the human mitochondrial ...
|
|
22955
|
Determinants of the dual cofactor specificity and substrate cooperativity of the human mitochondrial ...
|
|
22956
|
Determinants of the dual cofactor specificity and substrate cooperativity of the human mitochondrial ...
|
|
22957
|
Determinants of the dual cofactor specificity and substrate cooperativity of the human mitochondrial ...
|
|
22958
|
Determinants of the dual cofactor specificity and substrate cooperativity of the human mitochondrial ...
|
|
22959
|
Determinants of the dual cofactor specificity and substrate cooperativity of the human mitochondrial ...
|
|
22960
|
Determinants of the dual cofactor specificity and substrate cooperativity of the human mitochondrial ...
|
|
22961
|
Determinants of the dual cofactor specificity and substrate cooperativity of the human mitochondrial ...
|
|
22962
|
Determinants of the dual cofactor specificity and substrate cooperativity of the human mitochondrial ...
|
|
22963
|
Determinants of the dual cofactor specificity and substrate cooperativity of the human mitochondrial ...
|
|
22964
|
Determinants of the dual cofactor specificity and substrate cooperativity of the human mitochondrial ...
|
|
22965
|
Determinants of the dual cofactor specificity and substrate cooperativity of the human mitochondrial ...
|
|
22966
|
Determinants of the dual cofactor specificity and substrate cooperativity of the human mitochondrial ...
|
|
22967
|
Determinants of the dual cofactor specificity and substrate cooperativity of the human mitochondrial ...
|
|
22968
|
Determinants of the dual cofactor specificity and substrate cooperativity of the human mitochondrial ...
|
|
22969
|
Determinants of the dual cofactor specificity and substrate cooperativity of the human mitochondrial ...
|
|
22970
|
Determinants of the dual cofactor specificity and substrate cooperativity of the human mitochondrial ...
|
|
22971
|
Determinants of the dual cofactor specificity and substrate cooperativity of the human mitochondrial ...
|
|
22972
|
Determinants of the dual cofactor specificity and substrate cooperativity of the human mitochondrial ...
|
|
22973
|
Determinants of the dual cofactor specificity and substrate cooperativity of the human mitochondrial ...
|
|
22974
|
Determinants of the dual cofactor specificity and substrate cooperativity of the human mitochondrial ...
|
|
22975
|
Determinants of the dual cofactor specificity and substrate cooperativity of the human mitochondrial ...
|
|
22976
|
Determinants of the dual cofactor specificity and substrate cooperativity of the human mitochondrial ...
|
|
22977
|
Determinants of the dual cofactor specificity and substrate cooperativity of the human mitochondrial ...
|
|
22978
|
Determinants of the dual cofactor specificity and substrate cooperativity of the human mitochondrial ...
|
|
22979
|
Determinants of the dual cofactor specificity and substrate cooperativity of the human mitochondrial ...
|
|
22980
|
Determinants of the dual cofactor specificity and substrate cooperativity of the human mitochondrial ...
|
|
22981
|
Determinants of the dual cofactor specificity and substrate cooperativity of the human mitochondrial ...
|
|
22982
|
Determinants of the dual cofactor specificity and substrate cooperativity of the human mitochondrial ...
|
|
22983
|
Determinants of the dual cofactor specificity and substrate cooperativity of the human mitochondrial ...
|
|
22984
|
Determinants of the dual cofactor specificity and substrate cooperativity of the human mitochondrial ...
|
|
22985
|
Determinants of the dual cofactor specificity and substrate cooperativity of the human mitochondrial ...
|
|
22986
|
Determinants of the dual cofactor specificity and substrate cooperativity of the human mitochondrial ...
|
|
22987
|
Determinants of the dual cofactor specificity and substrate cooperativity of the human mitochondrial ...
|
|
22988
|
Determinants of the dual cofactor specificity and substrate cooperativity of the human mitochondrial ...
|
|
22989
|
Determinants of the dual cofactor specificity and substrate cooperativity of the human mitochondrial ...
|
|
22990
|
Determinants of the dual cofactor specificity and substrate cooperativity of the human mitochondrial ...
|
|
22991
|
Determinants of the dual cofactor specificity and substrate cooperativity of the human mitochondrial ...
|
|
22992
|
Mechanism of flavin reduction in class 2 dihydroorotate dehydrogenases
|
|
22993
|
Mechanism of flavin reduction in class 2 dihydroorotate dehydrogenases
|
|
22994
|
Mechanism of flavin reduction in class 2 dihydroorotate dehydrogenases
|
|
22995
|
Mechanism of flavin reduction in class 2 dihydroorotate dehydrogenases
|
|
22996
|
Mechanism of flavin reduction in class 2 dihydroorotate dehydrogenases
|
|
22997
|
Mechanism of flavin reduction in class 2 dihydroorotate dehydrogenases
|
|
22998
|
Mechanism of flavin reduction in class 2 dihydroorotate dehydrogenases
|
|
22999
|
Mechanism of flavin reduction in class 2 dihydroorotate dehydrogenases
|
|
23000
|
Mechanism of flavin reduction in class 2 dihydroorotate dehydrogenases
|




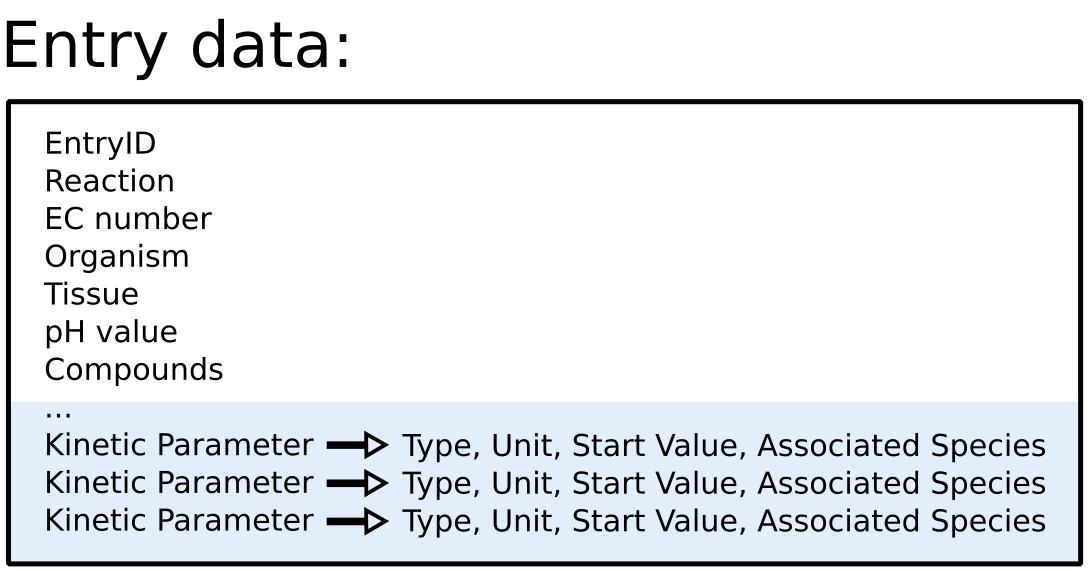
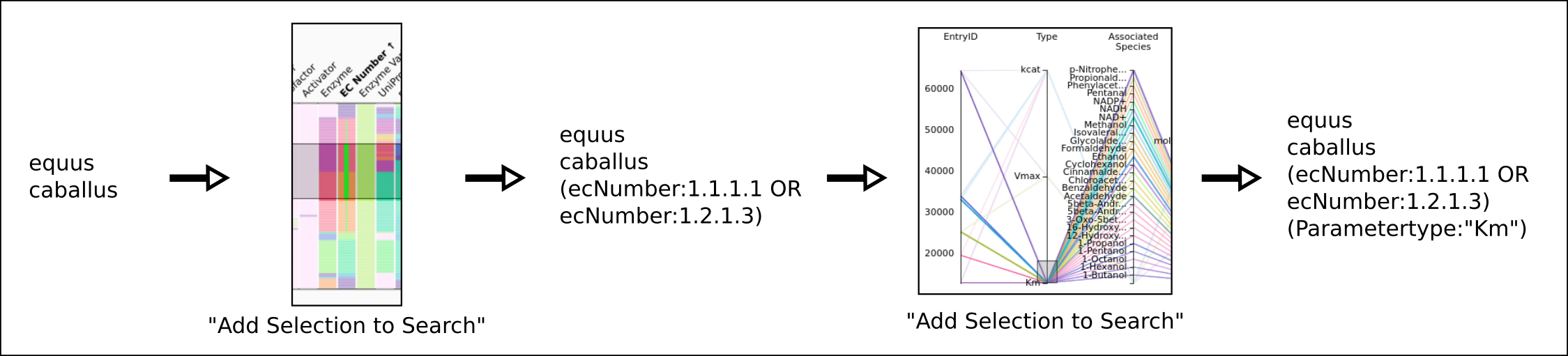 If the current search query is a refinement of the previous query "Use Previous Query (Go Back)" button can be used to perform the previous query again. Only one automatic step back is possible, but the query can be manually manipulated if desired.
If the current search query is a refinement of the previous query "Use Previous Query (Go Back)" button can be used to perform the previous query again. Only one automatic step back is possible, but the query can be manually manipulated if desired.